By confirming cognitive impairment before ordering amyloid blood tests, this two-step approach cuts false positives, streamlines referrals, and moves Alzheimer’s diagnosis closer to everyday clinics.
Study: Primary care detection of Alzheimer’s disease using a self-administered digital cognitive test and blood biomarkers. Image Credit: Orawan Pattarawimonchai / Shutterstock
In a recent study published in the journal Nature Medicine, researchers in Sweden and the United States assessed whether a brief self-administered digital cognitive battery, alone or combined with a blood-based amyloid biomarker panel, more accurately detects cognitive impairment and clinical Alzheimer’s disease (AD) in primary care than standard care.
Background
One in every three older adults worries about memory loss, yet many never receive a timely, accurate diagnosis. AD is the leading cause of dementia, driven by amyloid-beta (Aβ) deposition, tau aggregation, and neurodegeneration. Early stages like subjective cognitive decline (SCD) and mild cognitive impairment (MCI) often slip past brief paper tests such as the Mini-Mental State Examination (MMSE) or Montreal Cognitive Assessment (MoCA), especially in rushed clinics. Cerebrospinal fluid (CSF) or amyloid positron emission tomography (PET) can confirm pathology, but these methods are invasive, costly, or scarce. Blood tests like phosphorylated tau 217 (p-tau217) are promising, yet pretest probabilities vary widely in primary care, with amyloid positivity generally lower in SCD and higher in MCI or dementia, influencing positive predictive value (PPV). International guidance increasingly emphasizes two-step workflows that confirm impairment before biomarker testing. Further research should validate efficient, accurate, and scalable frontline pathways.
About the study
The investigators trained and validated BioCog, a tablet-based, self-administered test battery, across two Swedish cohorts. A secondary-care cohort from the BioFINDER-2 study (ClinicalTrials.gov NCT03174938) was used to build logistic-regression models and establish cutoffs; an independent primary-care cohort from 19 clinics in the BioFINDER-Primary Care study (NCT06120361) served for external validation. BioCog included a ten-word list (three immediate recalls, delayed recall, and delayed recognition), a cognitive processing-speed task, and items related to orientation to time. Objective cognitive impairment was defined using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS); in secondary care, a proxy for the RBANS from similar neuropsychological tests was used.
Model selection employed recursive feature elimination with Akaike Information Criterion (AIC) and receiver-operating-characteristic area under the curve (AUC) for performance. One-cutoff and two-cutoff schemes were predefined; the latter created an intermediate zone to be resolved by further evaluation. Two BioCog probability thresholds (0.332 and 0.769) were chosen to target 95% sensitivity and 95% specificity. Primary-care physicians (PCPs) performed standard evaluations, including MMSE, MoCA, computed tomography, and clinical judgment. A two-step workflow was tested: Step 1 involved BioCog to detect impairment; Step 2 involved a plasma panel (PrecivityAD2) generating the Amyloid Probability Score-2 (APS2) from Aβ42, Aβ40, p-tau217, and non-p-tau217. Sensitivity analyses used the Clinical Dementia Rating (CDR) global score ≥0.5 as an alternative impairment reference.
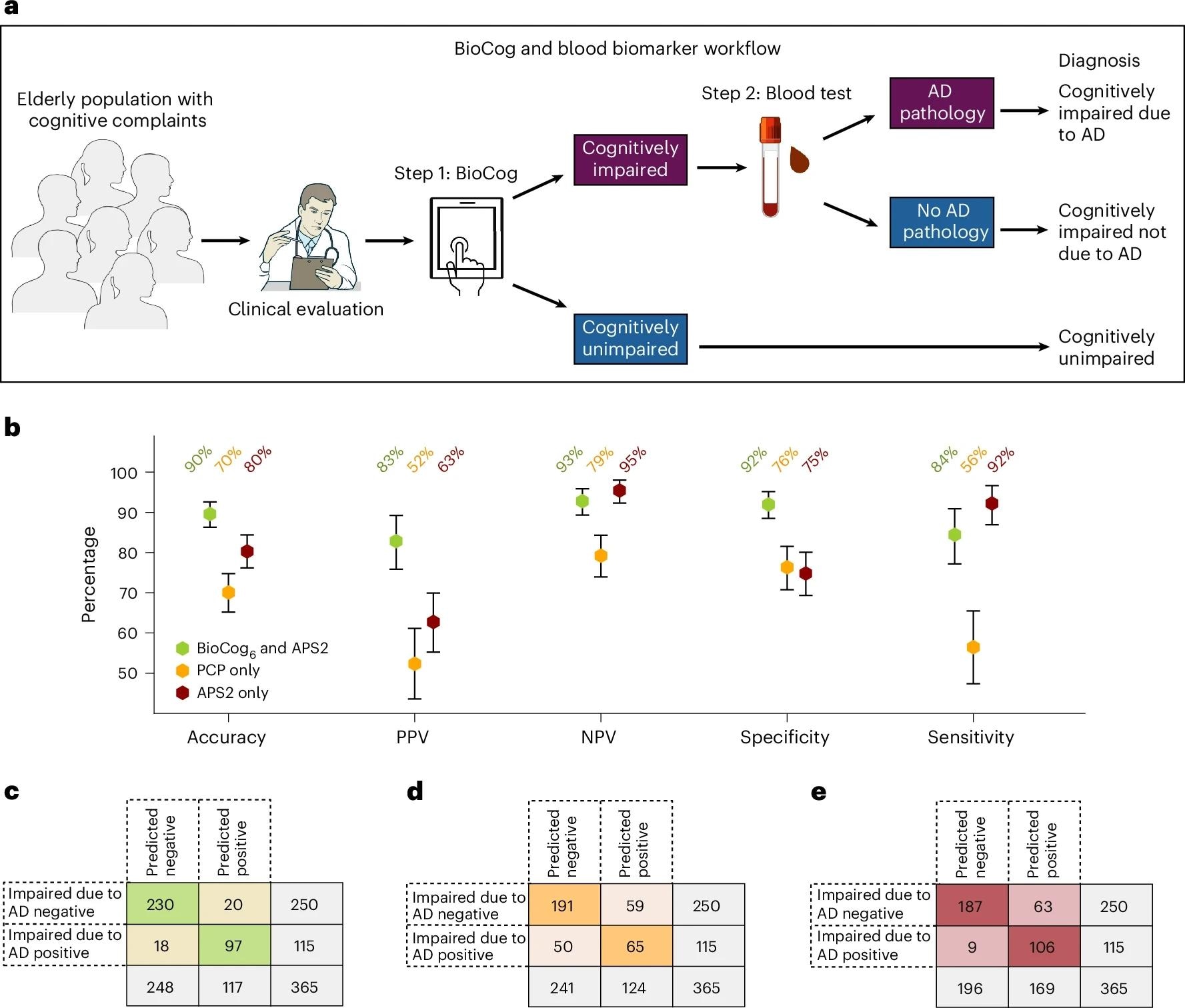
Comparing a digital testing and blood biomarker-based diagnostic workflow to the current standard clinical evaluation in the primary care cohort. Comparisons were made on a subset of individuals with all existing data available (n = 365; see Supplementary Table 17 for detailed population characteristics). a. Our proposed primary care two-step workflow consists of step 1, the detection of cognitive impairment using the BioCog, followed by step 2, a blood biomarker assessment to evaluate whether AD pathology is present in cognitively impaired individuals. b, Evaluation of the workflow using our BioCog6 model for step 1 and the plasma biomarker APS2 for step 2 (green). The workflow was compared against a standard clinical evaluation by PCPs in which the physician assesses both whether the patient had cognitive impairment (MCI or dementia) and whether the impairment was caused by AD (without any biomarkers) (PCPAD, orange), and against a workflow using only the plasma biomarker APS2 without any cognitive assessment (red). Error bars indicate 95% CI, with the center point corresponding to the mean value. Credit: Images in a adapted from NIAID NIH BIOART (https://bioart.niaid.nih.gov).
Study results
In secondary care (n=223; mean age 73 years), the best six-variable model (BioCog6: delayed recall, processing-speed correct, immediate recall performance, total time, age, and delayed recognition) achieved an AUC of 0.96 and 89% accuracy for objectively verified cognitive impairment at a single cutoff. A two-cutoff approach reached 96% accuracy with 18% of participants in an intermediate zone requiring follow-up. Psychometrics supported feasibility and validity: strong convergent correlations with paper analogs, weak divergent correlations with unrelated domains, an average completion time of about 11 minutes, and only around 2% reporting difficulty with instructions.
External validation in primary care (n=403; mean age 77 years) showed BioCog6 AUC 0.93 and 85% accuracy (positive predictive value [PPV] 87%, negative predictive value [NPV] 83%, sensitivity 88%, specificity 82%) using one cutoff, significantly outperforming PCP assessment (accuracy 73%) based on usual testing and imaging. With two cutoffs carried over from training, accuracy rose to 90% (PPV 91%, NPV 89%) with an 18% intermediate group. Head-to-head comparisons favored BioCog6 over MMSE, MoCA, Mini-Cog, and the Cambridge Neuropsychological Test Automated Battery (CANTAB) paired-associates learning, both in one-cutoff analyses and, where available, two-cutoff analyses, even after demographic adjustments.
Critically, the two-step workflow tailored for primary care, BioCog first, then blood testing only for those objectively impaired, identified biomarker-verified clinical AD (impairment due to AD by expert consensus with CSF or Aβ-PET support) with 90% accuracy (PPV 83%, NPV 93%, specificity 92%, sensitivity 84%). This surpassed PCP-only pathways (accuracy 70%) and outperformed blood testing alone (accuracy 80%), which showed an approximate 17% false-positive rate when used without an impairment filter. Using two cutoffs for both BioCog and APS2 raised accuracy to 95% with a larger intermediate zone (about 30%), emphasizing a trade-off between certainty and indeterminate referrals. Findings were accurate when substituting CDR ≥0.5 for RBANS (primary metrics dipped modestly but remained high) and when removing age from the BioCog model.
Interpretation centers on pretest probability: because only individuals with cognitive impairment (not SCD) are eligible for recently implemented Aβ-targeting immunotherapies, an efficient front-end filter that confirms impairment before blood biomarkers reduces false positives, improves PPV, and aligns with international recommendations and the World Health Organization’s preferred product profile for blood tests. Digital delivery standardizes administration, captures timing features beyond raw scores, and minimizes personnel time, advantages for busy clinics facing growing demand. However, the authors caution that BioCog must complement, not replace, clinical judgment and that validation in other languages, cultures, and longitudinal settings is still required.
Conclusions
This proof-of-concept demonstrates that a brief, self-administered digital cognitive battery can accurately identify objective cognitive impairment in primary care. When followed by a targeted blood-biomarker panel, it can diagnose clinical AD with substantially higher accuracy than the current standard-of-care. The stepwise “test-then-blood” approach enhances diagnostic certainty, reduces inappropriate referrals, and prioritizes candidates for Aβ-targeting therapies. While generalizability beyond Swedish settings and longitudinal utility need further study, the pathway is practical, scalable, and consistent with international guidance: confirm impairment first, then use blood biomarkers to infer amyloid pathology, bringing earlier, more confident diagnoses within reach of routine clinics.
Journal reference:
- Tideman, P., Karlsson, L., Strandberg, O., Calling, S., Smith, R., Midlöv, P., Verghese, P. B., Braunstein, J. B., Mattsson-Carlgren, N., Stomrud, E., Palmqvist, S., & Hansson, O. (2025). Primary care detection of Alzheimer’s disease using a self-administered digital cognitive test and blood biomarkers. Nat Med. DOI: 10.1038/s41591-025-03965-4, https://www.nature.com/articles/s41591-025-03965-4