Cell based assays are incredibly versatile and powerful tools used in the research lab. Some examples of cell based assays include analyses of cytotoxicity and cell viability that can be used to quickly detect changes in these parameters in real time. Such real-time detection makes it possible to identify the exact moment in time when an antiproliferative or cytotoxic change takes place. When a microplate reader is used to carry out these evaluations, it is possible to monitor multiple samples and concentrations on a single plate.
Promega’s CellTox Green Cytotoxicity Assay and RealTime-Glo® MT Cell Viability Assay were measured over three days. CLARIOstar, the latest microplate reader from BMG LABTECH, was used to maintain cell health (Figure 1). This device is fitted with an atmospheric control unit (ACU), enabling detection of time-dependent and dose-dependent effects on proliferation and cytotoxicity.

Figure 1. The CLARIOstar multi-mode microplate reader from BMG LABTECH
Principle of Assay
The bioluminescent assay, RealTime-Glo® MT Cell Viability Assay relies on the metabolic reducing potential of cells. pro-NanoLuc® substrate and NanoLuc® luciferase were introduced to cells in culture. Cells that are viable reduce the substrate, which then diffuses within the medium, where it is quickly used by NanoLuc® enzyme to create a luminescent signal that is proportional to the viable cell number, as shown in Figure 2.
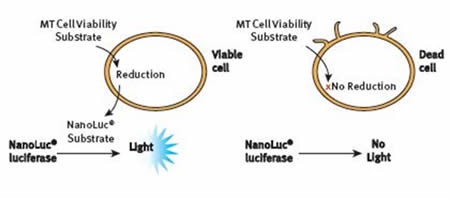
Figure 2. The RealTime-Glo® MT Cell Viability Assay Principle
The CellTox™ Green Cytotoxicity Assay is a non-activity based assay made up of a cell membrane impermeant dye that is excluded from viable cells. The dye only enters the cell when the cell membrane is compromised. Once inside the cell, the dye binds to DNA and becomes fluorescent. The fluorescent signal is proportional to the number of dead cells in culture (Figure 3).
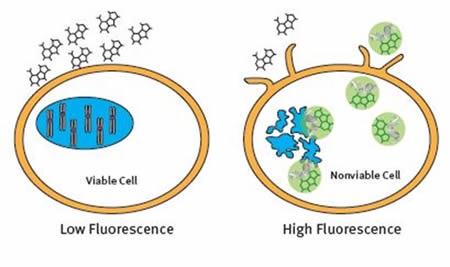
Figure 3. The CellTox™ Green Cytotoxicity Assay Principle
Materials and Methods
For the experiment, a number of materials were used including BMG LABTECH’s CLARIOstar microplate reader; Corning’s 384-well, white, clear bottom microplates; and Promega’s CellToxTM Green Cytotoxicity Assay and RealTime-GloTM MT Cell Viability Assay. K562 cells were plated in the 384-well microplates and treated with a panel of test compounds that have known effects on cytotoxicity and proliferation. The CLARIOstar equipped with ACU was used to incubate cells and to determine the changes in fluorescent and luminescence signals each hour for 72 hours.
The fluorescence instrument settings were as follows:
- Flashes per well: 50
- Method: bottom reading
- Optic settings: Excitation F: 482-16; emission F: 530-40
- No. of cycles: 73
- Dichroic: LP 504
- Gain + Focus: adjusted prior to test
- Cycle time: 60 min
Luminescence instrument settings are as follows:
- Cycle time: 60 min
- Measurement interval time: 1.0s
- Optic settings: No Filter
- Gain: 3500
- No. of cycles: 73
ACU settings include:
- O2: monitoring
- CO2: 5 %
- Target temperature: 37°C
Results and Discussion
BMG LABTECH’s CLARIOstar equipped with ACU was capable of fully sustaining the normal health and proliferation of untreated cells during the whole 72 hours, as shown in Figure 4.
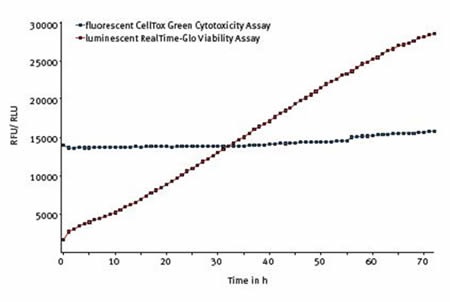
Figure 4. The Multiplexed RealTime-Glo® and CellTox™ Green assay. Average results of 10 replicates shows that cell viability increases and cytotoxicity is unchanged over 72 hours in untreated cells.
At first, cells that were treated with different concentrations of bosutinib, a tyrosine kinase inhibitor, displayed proliferation, although proliferation was suppressed by higher concentrations. Except for the lowest concentration, a change was observed after about 25 hours and cell viability started to decrease, as illustrated in Figure 5.
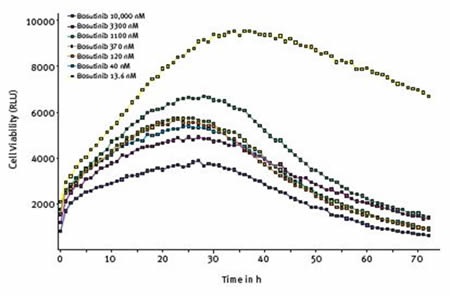
Figure 5. Effect of varying concentrations of bosutinib on cell viability assessed using RealTime-Glo® MT Cell Viability Assay. Average results of triplicates at the indicated concentrations of bosutinib.
Cytotoxicity was increased to some extent at all concentrations of bosutinib and starts to increase after approximately 30 hours, coincident with reduced cell viability (Figure 6).
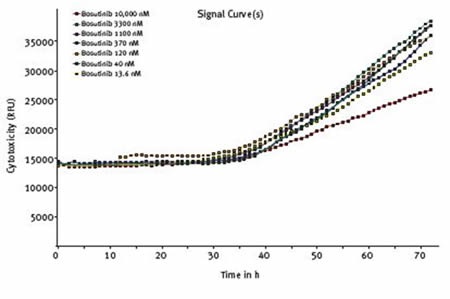
Figure 6. Effect of varying concentrations of bosutinib on cytotoxicity assessed using CellTox™ Green Cytotoxicity Assay. Average results of triplicates at the indicated concentrations of bosutinib.
Conclusion
BMG LABTECH’s CLARIOstar® integrated with ACU maintains cell health and proliferation, making it possible to achieve long term cell-based assays. In this analysis, cytotoxicity and cell viability were monitored over a period of 72 hours and the hourly evaluation of the effect of bosutinib is demonstrated here. The data reveal a distinct time dependence for cytotoxicity and a distinct time and dose dependence for cell viability.
Acknowledgement
Produced from articles authored by Carl Peters, BMG LABTECH, and Tracy Worzella, Promega.
About BMG Labtech

BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
All BMG LABTECH microplate readers are "Made in Germany" and are conceived, developed, assembled, and tested entirely at our headquarters in Germany.
Since our establishment in Offenburg, Germany in 1989, BMG LABTECH has expanded to offer a worldwide sales and support network with offices in the USA, UK, Australia, Japan and France. Our subsidiaries, regional offices and distributors are committed to bringing you innovative microplate reader technology with the quality and reliability you expect from a German company.
Our staff includes engineers and scientists from the fields of biology, biochemistry, analytical chemistry, and physics.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.