In the pharmaceutical industry, it is a standard practice to screen new candidate drugs, but at the same time, requirements on instrumentation and assays are ever increasing. Especially, as there is a need to further increase the number of samples that can be measured in a day.
Using highly condensed microplates, for example 1536-well microplates, can help meet these requirements. The equipment of choice must have the ability to measure these microplates with the fastest possible read time, while achieving the assay performance criteria such as Z´ value or S/B.
The microplate reader PHERAstar FSX from BMG LABTECH can address the requirements for the ultra-high throughput screening process. Thanks to the new TRF laser, the fastest read times can be achieved in 1536-well microplates. This article demonstrates the enhanced performances that can be achieved with the PHERAstar FSX, using two HTRF assays from Cisbio Bioassays.
Assay principle
The levels of myo-Inositol 1 phosphate (IP1) in cultured cells can be directly detected using the HTRF IP-One terbium cellular assay. IP1 will be produced by cells following a ligand binding to a Gq coupled receptor. Using this assay, modulation of the Gq pathway by GPCR agonists and antagonists can be studied. Figure 1 shows the assay principle.
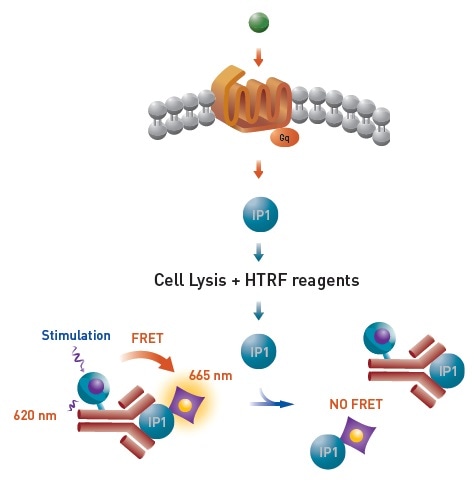
Figure 1. HTRF® IP-One terbium cellular assay principle. Image credit: BMG Labtech.
Here, the HTRF donor (Lumi4™-Tb cryptate) is used to label a monoclonal antibody specific for IP1. The HTRF acceptor-labeled IP1 competes with intracellular produced IP1 for the antibody binding sites.
There is an inverse relationship between the specific HTRF signal and the cellular IP1 concentration in the sample. The protein binding domain-peptide interaction is detected by the second assay. Figure 2 shows the assay principle.
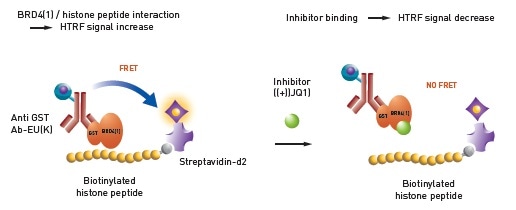
Figure 2. Histone – BRD4 interaction assay principle. Image credit: BMG Labtech.
An anti GST antibody containing the HTRF donor will attach to a GST tagged bromodomain module (BRD4) and a biotinylated histone peptide is attached to the HTRF acceptor.
A high HTRF signal can be observed as long as the histone peptide interacts with bromodomain. The interaction will be prevented by inhibitors, such as (+) JQ1, causing a decrease in HTRF signal.
Materials and methods
- EPIgeneousTM Histone/BRD4 Binding assay and HTRF IP-One assay from Cisbio
- PHERAstar FSX microplate reader from BMG LABTECH featuring flash lamp and TRF laser
- White 1536-well Hi-Base microplates from Greiner
- Certus nano liquid dispenser from Gyger
All cell culturing and pipetting processes are in line with Cisbio’s recommendations1,2. Using the Certus liquid handler, the reagent is dispensed into 1536-well microplates. After allowing the microplates to sit for the necessary incubation period, they are read on the PHERAstar FSX by using the instrument settings listed in the below table.
|
Detection Method
|
Time resolved fluorescence (dual emission)
|
|
Optic module
|
HTRF
|
|
Ecitation
|
337
|
|
Emission A
|
665
|
|
Emission B
|
620
|
|
Integration start
|
60 μs
|
|
Integration time
|
400 μs
|
|
Light source
|
Flash Lamp or Laser
|
|
Ratio multiplier
|
10000
|
Results and discussion
IP-One cellular assay
The concentration dependent effect of atropine on the IP-One level is shown in Figure 3.
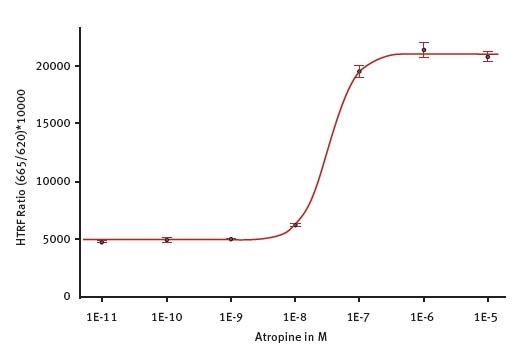
Figure 3. Antagonist atropine potency determination on CHO-M1 Gq coupled GPCR. Atropine inhibits the effect of a fixed concentration of acetylcholine agonist. Measurement was done on the PHERAstar FSX using the laser in flying mode (1 flash) to measure the HTRF signal. The 4-parameter fit was performed with the MARS Data Analysis software and was weighted using the formula 1/Y2. Image credit: BMG Labtech.
The TRF laser was used in 1 flash flying mode to perform the measurements. The R2 value shown by the 4-parameter standard curve fit is 0.9994. Using the MARS Data Analysis software, the IC50 value calculated from the fit is 33 nM, which is in line with the expectations.
Histone-BRD4-interaction assay
Figure 4 shows the inhibiting effect of the known inhibitor (+) JQ1 on the histone-BRD4 interaction.
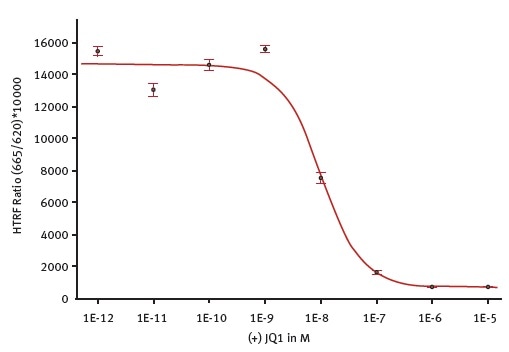
Figure 4. (+) JQ1 potency determination. Measurement was done on the PHERAstar FSX using the laser in flying mode (1 flash) to measure the HTRF signal. The 4-parameter fit was per- formed with the MARS Data Analysis software and was weighted using the formula 1/Y2. Image credit: BMG Labtech.
The above figures demonstrate the ability of the PHERAstar FSX to reliably measure the HTRF assays in 1536-well format. The HTRF measurement using the TRF laser in flying mode will allow users to read the microplates within 36 seconds and achieve excellent Z´ value. The data also reveals that using the TRF laser will result in higher S/B values than flash lamp measurements (Figure 5).
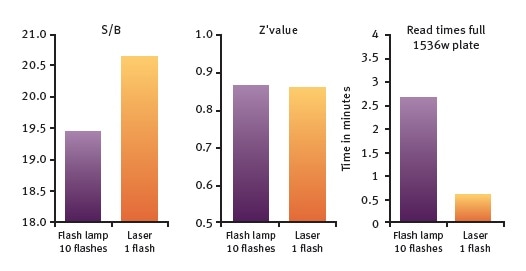
Figure 5. Histone-BRD4 interaction assay. Comparison of Flash lamp and laser measurements for S/B, Z´value and Read times. Image credit: BMG Labtech.
Conclusion
Rapid and reliable high throughput screening and compound follow up can be achieved when the PHERAstar FSX microplate reader is used in combination with Cisbio’s HTRF technology.
Acknowledgments
Produced from materials in originally authored by Franka Maurer1 ,Thomas Roux2 , Fanny Plénière2 and Najim Douayry2 from:
1 BMG LABTECH GmbH, Ortenberg, Germany
2 Cisbio Bioassays, Codolet, France
References
- https://www.cisbio.net/
- https://www.cisbio.com
About BMG Labtech

BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
All BMG LABTECH microplate readers are "Made in Germany" and are conceived, developed, assembled, and tested entirely at our headquarters in Germany.
Since our establishment in Offenburg, Germany in 1989, BMG LABTECH has expanded to offer a worldwide sales and support network with offices in the USA, UK, Australia, Japan and France. Our subsidiaries, regional offices and distributors are committed to bringing you innovative microplate reader technology with the quality and reliability you expect from a German company.
Our staff includes engineers and scientists from the fields of biology, biochemistry, analytical chemistry, and physics.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.