Genetically encoded, fluorescent biosensor-based live cell assays are advantageous in many ways over end point assays in cell lysates. They are able to monitor functional data about the location and timing of cellular responses in cells that are connected to diseases. Fluorescent biosensors, which have been used as basic research tools, have now been improved to facilitate detection on automated plate readers.1
In an earlier study, a live cell cADDIS assay from Montana Molecular for cAMP was employed to demonstrate the production of high Z’ values, characteristic of a robust screening assay2, using the CLARIOstar® microplate reader.
This article discusses the approach of detecting second messengers that are related to the Gq signaling pathway in live cells, using assays for PIP2, diacylglycerol (DAG), and Ca2+.
Here, the integration of spectrally distinct sensors into a single assay is shown to help detect two responses simultaneously. Using the CLARIOstar® in combination with a LVF monochromator or filters enables sensitive detection of such responses.
Assay principle
Packaging the biosensor within a modified baculovirus (BacMam) enables Montana Molecular’s biosensors to show optimal expression in mammalian cells. The mechanism of the DAG sensor is schematically represented in Figure 1, which shows the insertion of a circularly permuted fluorescent protein close to the DAG binding domain of protein kinase C. PIP2 and Ca2+ are detected using similar sensors.
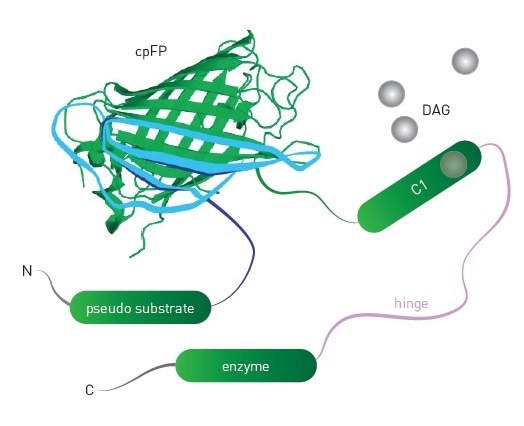
Figure 1. Schematic of DAG sensor. Upon binding DAG, the sensor undergoes conformational changes that lead to changes in fluorescence intensity of the engineered sensor. Image credits: BMG Labtech.
Each sensor is available in either a green or red version with the ability to be spectrally resolved. It is possible to detect the changes in two second messengers simultaneously by integrating green or red sensors into a single transduction step.
The two versions of DAG biosensors are Upward DAG (increasing in fluorescence) and Downward DAG (decreasing in fluorescence) and both of them show diacylglycerol increase in live cells.
Gq detection using DAG, PIP2, and Ca2+ sensors
An array of biosensors have been developed by Montana Molecular to unambiguously detect Gq signaling.3,4 hM1R mediated Gq signaling in response to Carbachol is shown in Figure 2. Here, the CLARIOstar® microplate reader was used to validate sensors for DAG, PIP2, and Ca2+.
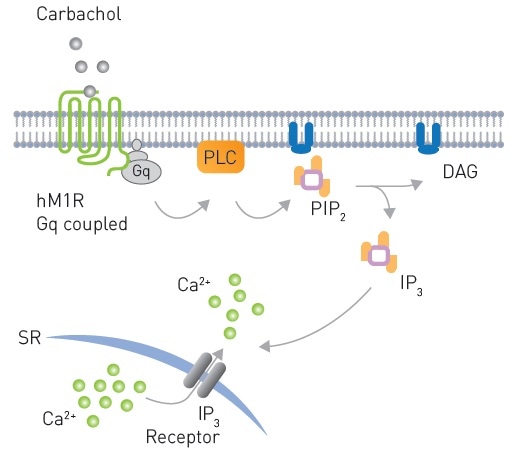
Figure 2. Signaling from a Gq coupled receptor to PLC after Carbachol stimulation leading to production of PIP2 and subsequently DAG production and Ca2+ mobilization. Image credits: BMG Labtech.
Materials and methods
- Montana Molecular DAG sensors, PIP2 sensors, R-GECO Ca2+ sensors packaged in BacMam
- HEK293 cells
- CLARIOstar® microplate reader from BMG LABTECH
- Greiner 96-well F bottom plates
Transduction of the indicated sensor packaged in BacMam was carried out on HEK293 cells in suspension. After subsequently plating the cells in 96-well microplates, sensor expression was carried out for 24 to 36 hours. 30 minutes before the experiment, media was replaced with PBS and cells were kept undisturbed at room temperature.
Instrument settings
|
Detection Mode
|
FI (well mode), bottom optic
|
|
Number of flashes
|
5
|
|
Scan mode
|
Orbital
|
|
Scan diameter (mm)
|
2
|
|
Gain / Focal height
|
Adjusted prior to test run
|
|
Interval time
|
3-11 seconds
|
Optical settings
|
|
Green
|
Red
|
|
Excitation
|
F 482-16
|
560-15
|
|
Dichroic
|
LP 504
|
auto580.5
|
|
Emission
|
F 530-40
|
618-49
|
Injection settings
|
Volume (μl)
|
50
|
|
Pump speed (μl/s)
|
85
|
|
Injection start time (s)
|
30
|
Results and discussion
Figure 3 presents the experimental results, showing the brilliant assay performance exhibited by the DAG sensor.
![DAG assay performance. Comparison of 14 replicates treated with Carbachol (white) or vehicle [PBS] (purple) indicates robust assay performance. Z’ = 0.8.](https://www.news-medical.net/image-handler/picture/2017/3/BMG3.3.jpg)
Figure 3. DAG assay performance. Comparison of 14 replicates treated with Carbachol (white) or vehicle [PBS] (purple) indicates robust assay performance. Z’ = 0.8. Image credits: BMG Labtech.
The results of a multiplexed experiment in response to Carbachol are shown in Figure 4, indicating the very rapid release of calcium and rapid DAG production following injection of the compound.
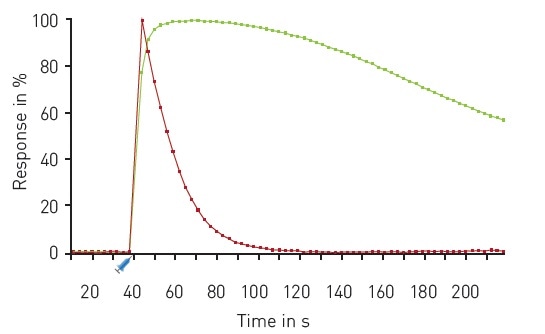
Figure 4. Multiplexed DAG and Ca2+ kinetics. Traces depict the average response to 30 µM Carbachol (n=16) expressed as 100% response. R-GECO (red); green DAG (green). Carbachol was dispensed using on-board reagent injectors at the 30 second time point as indicated. Image credits: BMG Labtech.
The results of another multiplexed experiment are shown in Figure 5, where a sensor for PIP2 and the Downward DAG sensor were used.
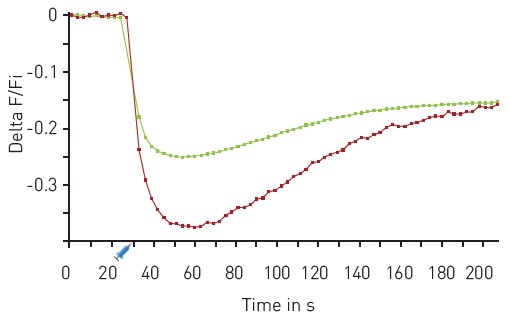
Figure 5. Multiplexed DAG and PIP2 kinetics. Traces depict average response to 30 µM Carbachol (n=18). Red DAG (red), green PIP2 (green). On board reagent injectors dispensed 50 µl of Carbachol after 30 seconds as indicated. Image credits: BMG Labtech.
Conclusion
The CLARIOstar® facilitates high sensitivity detection of Montana Molecular’s genetically encoded sensors. Spectrally resolved variants show simultaneous changes in several second messengers subsequent to Gq activation.
Acknowledgements
Produced from materials originally authored by P. Tewson1, S. Martinka1 , S. Tillo1 , T. Hughes1 , A.M. Quinn1 , C. Peters2 from:
1 Montana Molecular, Bozeman, MT
2 BMG LABTECH, Cary, NC
References
- Tewson, P., et al (2016) J. Biomol. Screen. 21:298-305
- Tewson, P., et al (2016) Real-Time Detection of Gs and Gi Signalling in Living Cells
- Ding, Y., et al (2015) Nature Methods 12:195-198
- Tewson, P., et al (2012) Simultaneous Detection of Ca2+ and Diacylglycerol Signaling in Living Cells
About BMG Labtech

BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
All BMG LABTECH microplate readers are "Made in Germany" and are conceived, developed, assembled, and tested entirely at our headquarters in Germany.
Since our establishment in Offenburg, Germany in 1989, BMG LABTECH has expanded to offer a worldwide sales and support network with offices in the USA, UK, Australia, Japan and France. Our subsidiaries, regional offices and distributors are committed to bringing you innovative microplate reader technology with the quality and reliability you expect from a German company.
Our staff includes engineers and scientists from the fields of biology, biochemistry, analytical chemistry, and physics.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.