A model organism in plant biology is the moss Physcomitrella patens. High rates of homologous recombination, a fast reproduction cycle, and the availability of efficient haploid protonema cell transformation methods make this model organism suitable to serve as an expression system for recombinant proteins.
The moss, as a eukaryotic organism, contains all significant posttranslational modification features necessary for biologically functional component production, making it a better option than bacterial production systems for example.
This article discusses the determination of total number of individualized viable moss cells per well using the autofluorescence, as represented by chlorophyll fluorescence, and comparison of this number with the relative number of freshly transformed, GFP-expressing cells.
BMG LABTECH’s CLARIOstar microplate reader has been used to perform all fluorescence scans and intensity measurements.
Material and methods
Moss protoplasts were generated from a bioreactor culture and then transformed with a vector containing the gene for GFP as fluorescent reporter, targeted to the nucleus. The protoplasts that were not transformed, served as control.
These cells were then serially diluted to achieve final theoretical concentrations of 225,000 down to 110 cells per well. All dilutions were carried out in duplicates in black microplates featuring transparent bottom wells.
A well containing medium only, served as a blank control. The following instrument conditions were used to set up the CLARIOstar for autofluorescence and GFP measurements:
|
Detection mode
|
Fluorescence intensity
|
|
Method
|
Bottom optic used
|
|
Scan Mode
|
Orbital averaging
|
|
Scan Diameter
|
3 mm
|
|
No. of flashes per well
|
8
|
|
Optic settings for autofluorescence LVF monochromator
|
475-30/680-20
|
|
Optic settings for wtGFP measurements LVF monochromator
|
385-12/510-20
|
Results and discussion
Determining the relative number of transformed moss cells using chlorophyll autofluorescence and GFP
Determining the minimum number of GFP-expressing cells required for measurement on the fluorescence reader is the aim of the study. In the future, it is possible to compare the relative expression rates of recombinant proteins present in various moss strains by using a prescribed number of moss cells.
The total number of viable cells in the microplate well can be easily calculated by utilizing the autofluorescence of moss cells. The far red emission of chlorophyll a and b pigments, present in the moss cells can be employed as a means for cell counting. The emission scan of untransformed moss cells and GFP-expressing moss cells is shown in Figure 1.
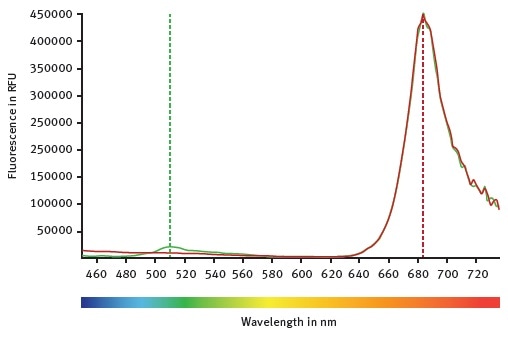
Figure 1. CLARIOstar emission scans between 450 and 740 nm while the excitation wavelength was set at 395 nm. Mock control moss cells are represented by the red curve. Emission of GFP expressing moss cells can be followed with the green curve. Image credits: BMG Labtech.
The emission scan of GFP-expressing moss cells and untransformed moss cells is shown in Figure 1. Both types of moss cells show a clear peak at around 684 nm, which indicates chlorophyll autofluorescence.
However, only GFP-expressing moss cells show the emission of wild type GFP at around 510 nm. Emission and excitation scans are then used to determine the optimal LVF monochromator chlorophyll wavelengths: emission at 680-20 and excitation at 475-30.
It is possible to calculate a standard curve from these measurements, revealing the relationship between the chlorophyll autofluorescence and the theoretical number of moss cells (Figure 2).
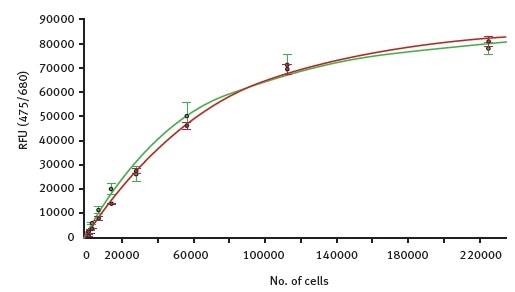
Figure 2. Fluorescence intensity values for autofluorescence were measured with the CLARIOstar in samples of increasing number of moss cells. Green curve shows dilution of GFP expressing cells while the red curve represents serial diluted cells that do not express GFP. Image credits: BMG Labtech.
The very high similarity between the two curves indicates the suitability of using autofluorescence as a tool to detect the total number of viable cells. Using the standard curves, the detection limit for moss cells via autofluorescence is found to be around 500 cells per well.
Well scan comparison of GFP expressing cells and mock control
Within the well, the protoplast distribution may not be even. This hypothesis was assessed by performing a well scan using samples containing, a medium-only control, untransformed moss cells, and GFP-expressing cells.
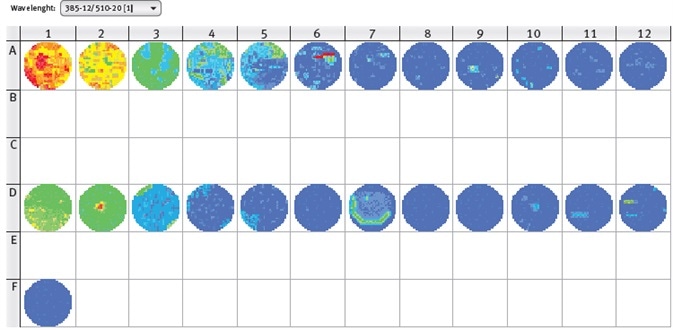
Figure 3. CLARIOstar well scan of GFP-expressing moss cells (line A), mock control (line D) and medium only control (Well F1). Excitation wavelength 385-12 nm, emission wavelength 510-20 nm. Image credits: BMG Labtech.
The results shown in Figure 3 clearly reveal the uneven distribution of the moss cells in the wells. Aggregates can be seen in some wells and would negatively affect the reliability of results from a single measurement in the center of well.
Therefore, the orbital averaging function is recommended to be used to perform the measurement on an orbit with definable diameter in the well and to display the average value of all orbit measurement points. Using this mode, more stable values can be obtained with reduced deviation of replicate wells.
As can be seen in Figure 3, fluorescence values of GFP-expressing moss cells are higher than the mock control, but the fluorescence values of the control increase with increasing number of cells.
These results conclude that the excitation wavelength of 385 nm also excites chlorophyll, confirming the need for a non-transformed control for background subtraction. Here, the transfection rate of GFP-construct into moss cells was around 20%.
With higher transfection rates, GFP-expressing moss cells and the mock control can be discriminated better, ultimately lowering the total number of cells necessary for measurements.
Conclusion
In this experiment, the CLARIOstar microplate reader was used to take GFP and chlorophyll emission spectra for optimization of measurement settings to buffer or special medium conditions. A standard curve can determine the total number of moss cells using chlorophyll fluorescence emission.
It is possible to detect moss cells down to 500 cells per well. Untransformed moss cells must be used as background control for analysis of GFP-expressing cells because some part of the fluorescence emission measured is related to chlorophyll.
Acknowledgments
Produced from materials originally authored by Nicola Krieghoff1, Benjamin Fode1 and Franka Maurer2 from:
1 Greenovation Biotech GmbH, Germany
2 BMG LABTECH GmbH, Ortenberg, Germany
About Greenovation
Greenovation develops plant-made next-generation therapeutics using its proprietary BryoTechnology platform. The company aims to optimize the production of highly efficient glycoproteins for the treatment of rare diseases.
About BMG Labtech

BMG LABTECH has been committed to producing microplate readers for more than twenty years. By focusing on the needs of the scientific community, the company’s innovative microplate readers have earned the company the reputation of being a technology leader in the field.
BMG LABTECH has developed a wide range of dedicated and multi-mode microplate readers for life sciences applications and high-throughput screening.
All BMG LABTECH microplate readers are "Made in Germany" and are conceived, developed, assembled, and tested entirely at our headquarters in Germany.
Since our establishment in Offenburg, Germany in 1989, BMG LABTECH has expanded to offer a worldwide sales and support network with offices in the USA, UK, Australia, Japan and France. Our subsidiaries, regional offices and distributors are committed to bringing you innovative microplate reader technology with the quality and reliability you expect from a German company.
Our staff includes engineers and scientists from the fields of biology, biochemistry, analytical chemistry, and physics.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.