Microtubules in neurons are stabilized by the protein, tau. These proteins are typically found in axons, however, they can be found in dendrites and other parts of neurons.
In patients with neurodegenerative diseases, known as tauopathies, tau becomes detached from microtubules and forms insoluble aggregates in their brain.
Tau and Alzheimer’s Disease
As the most common neurodegenerative disease, Alzheimer’s Disease (AD) affects 10% of seniors over the age of 65.1 The disease is named after a German scientist, Alois Alzheimer, who, when examining the brain of a deceased dementia patient in 1907, discovered tangled bundles of fibrils where neurons had previously been.2 AD is characterized by plaques containing amyloid beta and neurofibrillary tangles (NFTs) containing tau.
Tau Structure
Usually highly soluble3, tau has no well-defined secondary or tertiary structures.4 It is made up of four regions: the N-terminal region, the proline-rich domain, the repeat domain region, and the C-terminal region.5 On the whole, the protein is hydrophilic.6 It is thought that unbound tau in the cytoplasm have a “paper-clip” conformation, where the N and C terminals are close together.7
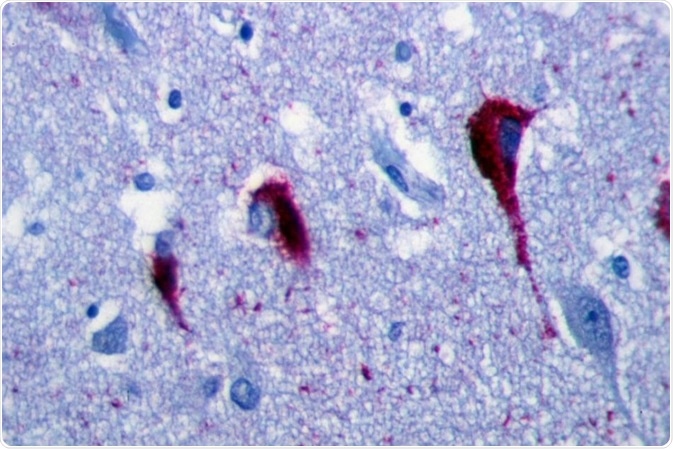
Immunohistochemistry of hippocampus of Alzheimer’s Disease patient shows neurofibrillary tangles. Image Credit: Patho [CC BY-SA 3.0], from Wikimedia Commons
Tau Isoforms
The MAPT gene, located on human chromosome 17, encodes tau. In the adult human brain, there are six isoforms. These are generated through alternative splicing of the tau gene. There are three isoforms with three microtubule binding repeat motifs (3R) and three with four microtubule binding repeat motifs (4R).8 Isoforms range 352-441 amino acids and from 60-74 kDa.5 Only the shortest isoform is expressed in the fetal human brain.9
Tauopathies
Tauopathies are neurodegenerative diseases where tau aggregates into neurofibrillary tangles (NFTs). AD, corticobasal degeneration, frontotemporal dementia, frontotemporal lobar degeneration, progressive supranuclear palsy, and Pick’s disease all come under this term.10 Huntington’s Disease also displays an increased amount of tau, including rod-like deposits.11
Tau Aggregation
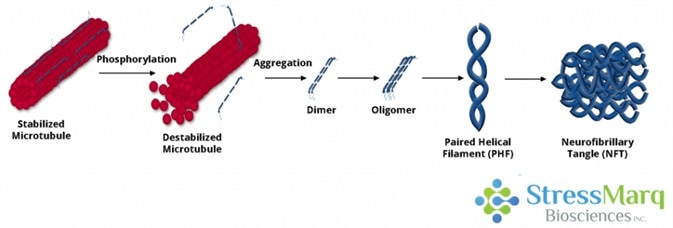
Tau dissociates from microtubules, leading to their destabilization. It then aggregates into oligomers, paired helical filaments, and ultimately neurofibrillary tangles. Image Credit: StressMarq Biosciences
Post-Translational Modifications
Tau can go through a number of post-translational modifications (PTMs) including phosphorylation, glycation, nitration, O-GlcNAcylation, acetylation, oxidation, ubiquitination, sumoylation, and methylation.12 Tau’s binding affinity to microtubules is regulated by these PTMs.13 There are high levels of ubiquitinated tau in AD and other tauopathies,12 and the tau function is impaired and aggregation promoted by lysine acetylation and other PTMs.10 In transgenic mouse models of tauopathies it is possible to detect acetylation of K280, however, this is not possible in control mouse brains.14
This suggests that it may be linked to tau pathology. Several post-translational modifications of tau occur, however, the most studied of these is phosphorylation.
Tau Hyperphosphorylation
Of the amino acids in tau, around 20% could potentially become phosphorylated.8 The microtubule-binding domain of tau is usually positively charged and, as such, attracts the negatively charged microtubules. It loses its positive charge when this domain becomes hyperphosphorylated and tau dissociates from the microtubules.
Tau is then no longer able to assemble or stabilize them. It is possible that the subsequent neurodegeneration is a result of a loss in microtubule function, hyperphosphorylated tau being toxic to neurons, or a combination of these effects.15
Paired Helical Filaments
Dimers can be formed through the combination of two hyperphosphorylated tau monomers.8 Known as dimerization, these interactions between hexapeptides in repeats 2 and 3 can lead to subsequent oligomerization.16 Following this, oligomers aggregate into PHFs. These have a twisted double-helical ribbon structure.17
Tau is more negatively charged than monomeric tau when contained within PHFs. It is, therefore, not able to bind tubulin or stabilize microtubules as effectively.18 If the microtubules are not stabilized then the microtubule network decays and neurons cease to function.
Neurofibrillary Tangles
A filamentous, insoluble aggregate of tau, NFTs have an increased beta-sheet structure19 and consist of aggregated PHFs. There is an association between dementia severity20 and the degree of NFT deposition in the brain as well as an association with neuron death.21
Neuropil threads and neuritic plaques are two other forms of tau aggregates in AD patients, which also induce neuron degeneration.22 Research suggests, however, that filamentous and fibrillary tau could have some neuroprotective effects and that the most toxic form of tau is soluble, hyperphosphorylated tau oligomers.23
Tau Propagation
“Prion-like propagation” is one suggested mechanism for the propagation of tau pathology. Here tau ‘seeds’ or fibrils are transferred from a donor cell to a recipient cell, where they recruit endogenous tau proteins onto their ends.24 Tau fibrils seemingly spread similarly to prions. However, it is unclear whether tau and prions share other properties, such as human-to-human infection.25
Tau Preformed Fibrils
The aggregation of tau in both cultured cells25 and living animals13 can be induced by preformed fibrils (PFFs) by recruiting soluble monomers to form insoluble fibrils. The introduction of minute quantities of tau PFFs into tau-expressing cells may cause a great amount of tau to be recruited into “filamentous inclusions resembling NFTs.”27 Synthetic tau PFFs may also enter non-neuronal cells and recruit tau into NFTs.28
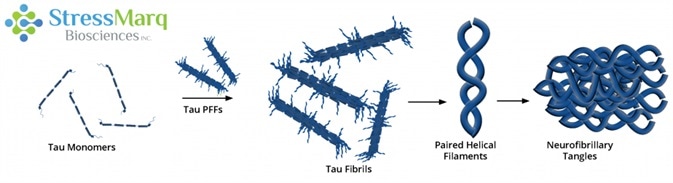
Tau PFFs can act as seeds, recruiting monomers into larger fibrils. Image Credit: StressMarq Biosciences
Tau Truncation
Full-length tau PFFs, have been shown in some research studies, to seed aggregation more effectively than truncated K18 tau in primary neurons.28 However, severe but reversible neurotoxicity in mice was shown to be induced by co-expression of truncated and full-length tau.29 This could be as a result of the formation of soluble, non-filamentous, high-molecular weight oligomers that are toxic to neurons.29
Tau and Alpha Synuclein
The formation of Lewy bodies occurs in more than 50% of AD cases. What’s more, the brains of patients with Parkinson’s Disease and Dementia with Lewy bodies often display amyloid-beta plaques and NFTs.30 Alpha synuclein and tau promote one another’s fibrillization in vitro,31 and tau fibrils colocalize with alpha synuclein fibrils in Lewy bodies.32 Tau aggregation in vitro may be induced through the contribution of alpha synuclein PFFs to tau phosphorylation.33 In both tauopathies and synucleinopathies, tau and alpha synuclein interaction and fibrillization may occur.
Therapeutic Approaches
Targeting the tau protein is involved in several approaches to potential treatments for AD and other tauopathies. Tau hyperphosphorylation is controlled by protein kinases and phosphatases.34 As such, treating tau may involve inhibiting tau kinases, restoring protein phosphatase 2A, or targeting the O-glycosylation of tau.35 Another option is to target neuroinflammation, as it plays a role in tau propagation.15 It has also been shown that immunizing mice with tau antibodies can slow the disease progression,36 suggesting that immunotherapy could be a potential treatment option.
References and Further Reading
- Facts and Figures. Retrieved from https://www.alz.org/alzheimers-dementia/facts-figures
- Alzheimer, A. Z. Psych.-Gerichtl. Med. 64, 146–148 (1907)
- Irwin, D. J., Cohen, T. J., Grossman, M., Arnold, S. E., Xie, S. X., Lee, V. M., & Trojanowski, J. Q. (2012). Brain, 135(3), 807-818.
- Weinreb, P. H., Zhen, W., Poon, A. W., Conway, K. A., & Lansbury, P. T. (1996). Biochem, 35(43), 13709-13715.
- Maina, M. B., Al-Hilaly, Y., & Serpell, L. (2016). Biomol, 6(1), 9.
- Avila J, Jimenez JS, Sayas CL, Bolos M, Zabala JC, Rivas G, Hernandez F (2016). Front Aging Neurosci 8:262.
- Jeganathan S, von Bergen M, Brutlach H, Steinhoff HJ, Mandelkow, E (2006). Biochem, 45:2283–2293.
- Götz, J., Gladbach, A., Pennanen, L., Eersel, J. V., Schild, A., David, D., & Ittner, L. M. (2010). Biochimica Et Biophysica Acta (BBA) – Molecular Basis of Disease, 1802(10), 860-871.
- Jovanov-Milošević, N., Petrović, D., Sedmak, G., Vukšić, M., Hof, P. R., & Šimić, G. (2012). Intl J Bioc & Cell Bio, 44(8), 1290-1294.
- Spillantini, M. G., & Goedert, M. (2013). The Lancet Neuro, 12(6), 609-622.
- Fernandez-Nogales M, Cabrera JR, Santos-Galindo M, Hoozemans JJ, Ferrer I, Rozemuller AJ, Hernandez F, Avila J, Lucas JJ (2014) Nat Med 20:881–885.
- Park, S., Lee, J. H., Jeon, J. H., & Lee, M. J. (2018). BMB Reports, 51(6), 265-273.
- Iba, M., Guo, J., McBride, J. D., Zhang, B., Trojanowski, J. Q., Lee, V.M. (2013). J Neurosci. 33(3): 1024-1037.
- Cohen, T. J., Guo, J. L., Hurtado, D. E., Kwong, L. K., Mills, I. P., Trojanowski, J. Q., & Lee, V. M. (2011). Nat Comm, 2(1).
- Gong, C., & Iqbal, K. (2008). Cur Med Chem, 15(23), 2321-2328.
- Guo, T., Noble, W., & Hanger, D. P. (2017). Acta Neuropathologica, 133(5), 665-704.
- Crowther, R. A. (1991). Proc Nat Ac Sci, 88(6), 2288-2292.
- Ksiezak-Reding, H., & Yen, S. (1991). Neuron, 6(5), 717-728.
- Barghorn, S., Davies, P., & Mandelkow, E. (2004). Biochem, 43(6), 1694-1703.
- Arriagada, P. V., Growdon, J. H., Hedley-Whyte, E. T., and Hyman, B. T. (1992). Neurology 42, 631–639
- Rajendran, L., Honsho, M., Zahn, T. R., Keller, P., Geiger, K. D., Verkade, P., and Simons, K. (2006) Acad. Sci. U.S.A. 103, 11172–11177
- Lim, S., Haque, M. M., Kim, D., Kim, D. J., & Kim, Y. K. (2014). Comp Struct Biol J, 12(20-21), 7-13. doi:10.1016/j.csbj.2014.09.011
- Cowan, C. M., & Mudher, A. (2013). Frontiers in Neuro, 4.
- Kfoury, N., Holmes, B. B., Jiang, H., Holtzman, D. M., and Diamond, M. I. (2012) Chem. 287, 19440−19451.
- Hyman, B. (2014). Neuron, 82(6), 1189-1190.
- Frost, B., Jacks, R. L., & Diamond, M. I. (2009). J Biol Chem, 284(19), 12845-12852.
- Guo, J., Lee, V.M. (2011). J Biol Chem. 286(17):15317-31.
- Guo, J. L., & Lee, V. M. (2013). FEBS Letters, 587(6), 717-723.
- Ozcelik, S., Sprenger, F., Skachokova, Z., Fraser, G., Abramowski, D., Clavaguera, F., . . . Winkler, D. T. (2016). Mol Psychiatry, 21(12), 1790-1798.
- Galpern, W. R., & Lang, A. E. (2006). Ann Neuro, 59(3), 449-458.
- Giasson, B. I. (2003). Science, 300(5619), 636-640.
- Ishizawa, T., Mattila, P., Davies, P., Wang, D., & Dickson, D. W. (2003). J Neuropath & Expt Neuro, 62(4), 389-397.
- Waxman, E. A., & Giasson, B. I. (2011). J Neuro, 31(21), 7604-7618.
- Hanger DP, Anderton BH, Noble W (2009) Trends Mol Med. 15:112–119.
- Gong, C.X., Iqbal, K. (2008). Chem. 15:2321–2328.
- Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM (2007) J Neurosci 27:9115–9129.
Acknowledgments
Produced from materials originally authored by Patricia Thomson from StressMarq Biosciences Inc.
About StressMarq Biosciences
Established in 2007, StressMarq Biosciences Inc. is a supplier of life science products that operates out of Victoria, Canada with a small, but dedicated group of scientists. Headed by our CEO and President Dr. Ariel Louwrier, StressMarq provides the research community with high-quality reagents backed with rigorous quality control data, expert scientific support, and fast international delivery.
“Discovery through partnership, Excellence through quality”
With over 7,000 products, our growth can be attributed to the continual production of cutting edge research products. Our diverse portfolio of primary antibodies, antibody conjugates, proteins, immunoassay kits and small molecules bridges across the life sciences, including products for cancer research, cardiovascular disease, cell signaling and neuroscience. To aid research worldwide, StressMarq has an extensive network of international distributors that allow us to supply reagents to over 50 countries.
In the years to come, StressMarq will continue to aid life science research by providing “Discovery through partnership, and Excellence through quality”.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.