COVID-19 vaccines developed in response to the ongoing pandemic have shown significant efficiency in controlling SARS-CoV-2 infection rate and related mortality at the initial phase of worldwide vaccine deployment. However, with the emergence of immunologically more aggressive viral variants such as the delta and omicron, a decline in vaccine efficacy has been observed worldwide.
The majority of COVID-19 vaccines comprise a two-dose regimen administered at a fixed interval. However, given the waning vaccine efficacy, a third booster has been introduced in the mass vaccination programs. Studies conducted at real-world setups have shown that the third booster is highly effective against delta and omicron infections and related disease severity, hospitalization, and death.
In the current study, scientists have evaluated the long-term efficacy of the third booster dose of mRNA-based COVID-19 vaccine (Pfizer/BioNTech) in older adults.
Study design
The study was conducted on 36 people aged 61-81 years. The participants received two primary doses of the Pfizer vaccine at an interval of 21 days. The third booster dose was administered 189-270 days after the first dose.
Blood samples were collected from the participants at two and five months after primary vaccination and one and four months after the booster vaccination. The samples were analyzed for SARS-CoV-2 spike-specific antibodies, memory B cell response, and T cell response.
Antibody response induced by mRNA COVID-19 vaccine
The antibody level analyzed two months after the primary vaccination revealed a significant induction in response against wild-type SARS-CoV-2. However, a considerable proportion of participants (25%) showed low antibody response.
Regarding viral variants, a significantly lower antibody response was observed against tested variants (alpha, beta, gamma, kappa, delta, delta plus, and omicron) at two months compared to that against the wild-type virus. The response was lowest against the omicron variant.
The antibody response against wild-type virus remained unchanged at five months post-primary vaccination. However, a significant reduction in antibody response against all tested variants was observed at this timepoint. The percentage of low antibody responders increased from 25% to 71-81%, especially for the variant-specific antibody response.
A significant induction in antibody response was observed against wild-type virus and tested variants at one-month post-booster vaccination. Even low responders showed a similar induction. However, a significant reduction in antibody response was observed at four months compared to one month.
Despite waning vaccine efficacy, the antibody response observed at four months post-booster vaccination was significantly higher than at five months post-primary vaccination.
Memory B cell and T cell response induced by mRNA COVID-19 vaccine
A significant induction in memory B cell response was observed one month after booster vaccination, which remained unchanged after four months. The response at four months post-booster vaccination was higher than at five months post-primary vaccination.
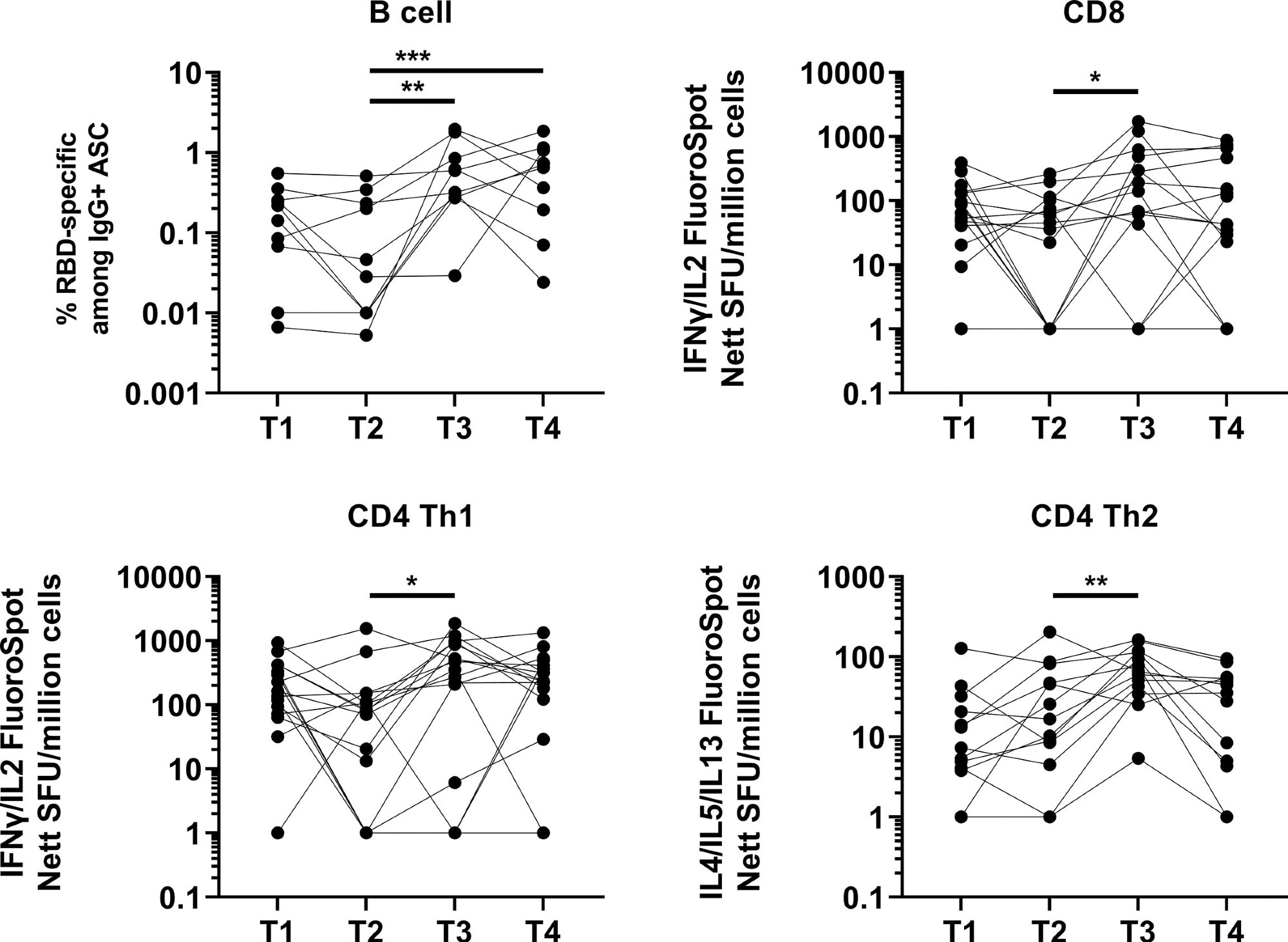 Induction and waning of memory B and T cell response against Spike following booster vaccination. RBD-specific memory B (N=10), Spike-protein-specific CD8 (N=16), CD4 Th1 (N=16) or CD4 Th2 (N=13) responses. The frequency of IgG RBD-specific memory B cells among total IgG antibody-secreting cells (ASCs) is presented for memory B cell response. For CD8, CD4 Th1, and CD4 Th2 responses, data presented are spot forming units (SFU) per million PBMC from paired samples at four time points. Each data point represents the normalized mean spot count from duplicate wells after subtraction of medium-only control. To compare between time points, Friedman tests and post hoc tests using Dunn’s multiple comparison tests were used. *, P-value <0.05; **. P-value <0.01; ***, P-value <0.001.
Induction and waning of memory B and T cell response against Spike following booster vaccination. RBD-specific memory B (N=10), Spike-protein-specific CD8 (N=16), CD4 Th1 (N=16) or CD4 Th2 (N=13) responses. The frequency of IgG RBD-specific memory B cells among total IgG antibody-secreting cells (ASCs) is presented for memory B cell response. For CD8, CD4 Th1, and CD4 Th2 responses, data presented are spot forming units (SFU) per million PBMC from paired samples at four time points. Each data point represents the normalized mean spot count from duplicate wells after subtraction of medium-only control. To compare between time points, Friedman tests and post hoc tests using Dunn’s multiple comparison tests were used. *, P-value <0.05; **. P-value <0.01; ***, P-value <0.001.
Regarding cellular immune response, a robust and durable T cell response was observed at five months post-primary vaccination compared to two months.
After one month of booster vaccination, a significant induction in CD4 type 1 T helper cell response was observed. After booster vaccination, only 12% and 6% of participants developed CD8 and CD4 type 1 T helper cell responses, respectively.
The overall T cell response remained unchanged four months after booster vaccination. However, about 50%, 43%, and 84% of participants showed a decline in CD8 type 1 T helper cell, CD4 type 1 T helper cell, and CD4 type 2 T helper cell responses, respectively. In addition, after four months of booster vaccination, about 25%, 18%, and 53% of participants showed lower CD8 type 1 T helper cell, CD4 type 1 T helper cell, and CD4 type 2 T helper cell responses, respectively, compared to after five months of primary vaccination.
Study significance
The study reveals that the third booster dose of the mRNA COVID-19 vaccine is capable of inducing a robust but transient immune response in older adults. However, a rapidly waning booster vaccination efficacy highlights the need for administering an additional fourth dose to protect older people from severe COVID-19.