This article is based on a poster originally authored by Maryam Ahmadi, Elena Shvets, Frank Gesellchen, Jitender Bisht, Joshua Stewart, Manju Namdeo, Marco Stefano Torrisi, Sophie Davies, and Richard Hammond.
Antibody drugs are at the forefront of pharmaceutical innovation; however, extended workflows and rising costs in their discovery and development pose significant challenges.
Fluidic Sciences and Sphere Bio’s Cyto-Mine® platform combines picodroplet technology, microfluidics, and optics to address limitations in both Antibody Discovery (AbD) and Cell Line Development (CLD), helping accelerate therapeutic development.
The next-generation Cyto-Mine® platform, Cyto-Mine® Chroma, is an advanced microfluidic platform featuring multi-laser, single-cell analysis capabilities. These enhancements enable high-throughput, multiplex functional screening of millions of cells in a single day.
Experimental results confirm Cyto-Mine® Chroma’s ability to sort and dispense cells with high precision, using fluorescence signals and sequential gating. The system achieves over 90 % accuracy in isolating cells based on productivity and cellular markers from mixed populations.
In a study simulating an AbD workflow, Cyto-Mine® Chroma successfully isolated rare cells secreting antigen-specific antibodies with high precision. Furthermore, by incorporating a viability dye alongside IgG-specific probes, the platform isolated CHO cells producing human IgG4, using both productivity and viability as selection criteria, without compromising cell performance.
These findings underscore the powerful multiplexing abilities of Cyto-Mine® Chroma, establishing it as a vital tool for isolating specific cell populations in both CLD and AbD workflows.
Materials and methods
Instrument:
Cyto-Mine® Chroma builds on its predecessor’s capabilities as a fully automated single-cell analysis system that encapsulates, analyzes, sorts, and dispenses viable single cells (Figure 1a). The platform has been upgraded with four lasers and four detectors (Figure 1b), enhancing its performance and enabling multiplexing within picodroplets (Figure 1c).
Figure 1d summarizes the integrated stages of the Cyto-Mine® Chroma process. Cells and assay reagents are encapsulated into picodroplets and incubated at optimal temperature to promote target protein secretion. The secreted protein accumulates within each picodroplet, where it is captured. Cells producing the desired protein are detected during the sorting stage and are then dispensed into tissue culture plates.
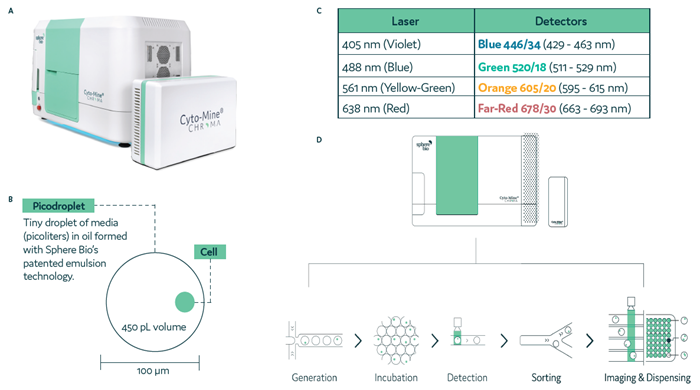
Figure 1. Cyto-Mine® Chroma, the next-generation single-cell analysis platform. Image Credit: Sphere Fluidics Limited
Study 1) Accuracy of sequential gating during sorting:
To assess the accuracy of sorting and gating, four cell samples were labeled with distinct cell membrane dyes: CellTracker™ Green CMFDA (Green, CT-Green), CellTracker™ Orange CMTMR (Orange, CT-Orange), eBioscience™ Cell Proliferation Dye eFluor™ 450 (Blue, eFL450), and eBioscience™ Cell Proliferation Dye eFluor™ 670 (Red, eFL670).
The stained cells were mixed and loaded into the Cyto-Mine® Chroma platform. Cells were gated for a single dye while excluding others, then dispensed.
Gating and sorting accuracy was determined using a fluorescence microscope. This procedure was repeated for all four dyes (Figure 2a, Figure 2b).
Study 2) Detecting antibody secretion:
To identify antibody-secreting cells, two hybridoma cell lines were labeled with different dyes (eFL450 [Blue] and eFL670 [Far Red]) and mixed with anti-mouse IgG FRET probes (Donor: Dylight488 [DL488, Green]; Acceptor: Dylight594 [DL594, Orange]).
Cells were loaded into Cyto-Mine® Chroma and sequentially gated - first for FRET+ response, then for the presence of Blue cells.
The same gating logic was used during cell dispensing. Accuracy of gating, sorting, and dispensing was evaluated via fluorescence microscopy (Figure 3a, Figure 3b).
Study 3) Detecting antibody binding to its antigen:
To assess antigen-specific antibody binding, antibody-secreting hybridoma cells (labeled with eFL450 [Blue]) were mixed with target cells expressing a specific antigen (labeled with eFL670 [Far Red]). Binding was detected using a detection antibody labeled with AF488 (Green).
Cells were loaded onto the Cyto-Mine® Chroma, and droplets containing localized antibody-secreting cells (ASC), target cells, and detection antibody were sorted. Sequential gating was applied for dispensing. Gating, sorting, and dispensing accuracy was assessed using a fluorescence microscope (Figure 4a, Figure 4b).
Study 4) Detecting apoptosis and dead cells in droplets:
A CHO cell line secreting human IgG4κ antibodies was mixed with Cyto-Cellect® reagents (Donor conjugated with AF488 [Green], Acceptor with AF594 [Orange]) and a viability dye conjugated to AF670 (Far Red). Cells were processed using the Cyto-Mine® Chroma platform.
Droplets were gated for FRET+ viable cells before dispensing. Accuracy of gating and sorting was analyzed using FlowJo software (v10.10.0; Figure 5a, Figure 5b).
Results
Study 1) Accurate sorting and gating of encapsulated live cells
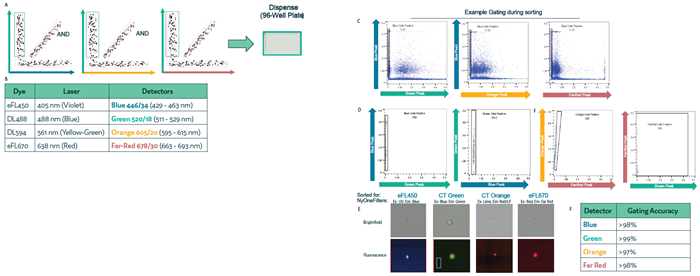
Figure 2. Sorting and gating accuracy using Cyto-Mine® Chroma. Four cell samples, each labeled with a different membrane dye, were mixed and loaded into the Cyto-Mine® Chroma platform. During sorting, cells were gated for a single dye while excluding all others, followed by dispensing. (a) Schematic illustration of the gating process during sorting. (b) Summary of fluorochromes used in the assay. (c) Example gating strategy for sorting Blue-labeled cells and the corresponding population after dispensing. (d) Dot plot showing the dispensed cell populations for Blue, Green, Orange, and Far Red-labeled cells, based on the gating strategy shown in (c). (e) Representative images of dispensed cells following sorting and dispensing of specific cell populations. (f) Microscopy analysis of dispensed cells used to assess the accuracy of gating during sorting. Image Credit: Fluidic Sciences and Sphere Bio
Study 2) Single-cell detection and isolation of antibody-secreting cells
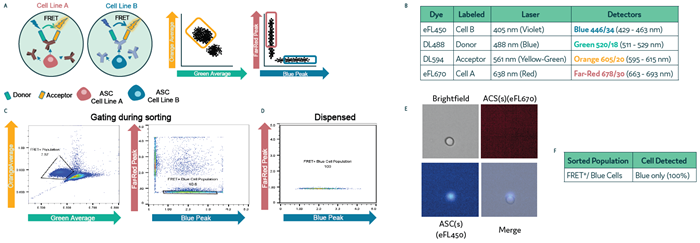
Figure 3. Isolation of antibody-secreting cell subpopulations on Cyto-Mine® Chroma. Two differentially labeled cell populations were pre-mixed with anti-mouse IgG FRET probes and loaded into the Cyto-Mine® Chroma platform. (a) Schematic representation of the assay reaction and gating strategy used during sorting. (b) Summary of fluorochromes used in the assay. (c) Cells were gated first for FRET+, followed by gating specific to Blue-labeled cells. (d) Profile of the dispensed cell populations. (e) Representative images of dispensed cells following sorting and dispensing of the targeted population. (f) Microscopy data of dispensed cells used to assess gating accuracy during sorting. Image Credit: Fluidic Sciences and Sphere Bio
Study 3) Isolation of antibody-secreting cells through antigen-specific binding
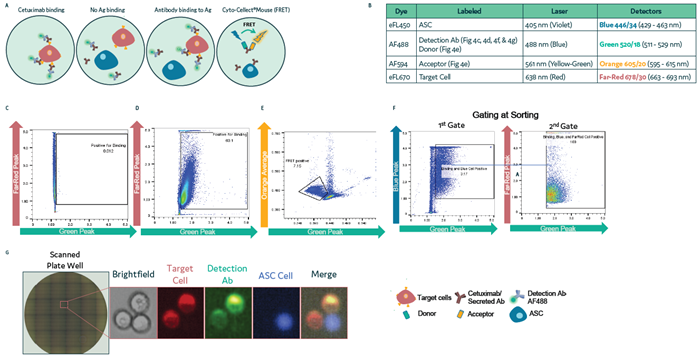
Figure 4. Isolation of ASC via a surface binding assay using Cyto-Mine® Chroma. (a) Schematic diagram illustrating the assay reaction and gating strategies used during sorting. (b) Summary of fluorochromes used in the assay. (c–d) A431 target cells labeled with eFL670 were mixed with anti-hIgG-AF488 antibody either in the absence (c) or presence (d) of Cetuximab. Cells were loaded onto Cyto-Mine® Chroma and gated for Far Red and Green signals during sorting. (e) IgG secretion of ASC measured using Cyto-Cellect® Mouse FRET probes. (f–g) ASC labeled with eFL450 were combined with eFL670-labeled target cells in the presence of AF488 detection antibody. Droplets were gated during sorting (f) and dispensed into 96-well plates. (g) Dispensed cells were visualized using fluorescence microscopy. Image Credit: Fluidic Sciences and Sphere Bio
Study 4) Differentiating FRET+ viable cells from apoptotic or dead cells
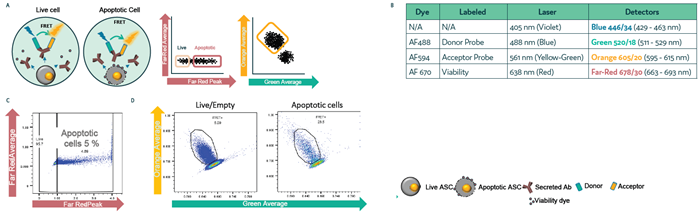
Figure 5. Differentiating FRET+ dead/dying cells from live cells in picodroplets. CHO cells secreting hIgG4 antibodies were mixed with Cyto-Cellect® probes and a viability dye, then introduced into the Cyto-Mine® Chroma platform and incubated for 1 hour. Cells were gated based on apoptosis and FRET signal. (a) Schematic diagram of the assay reaction and gating strategy used during sorting. (b) Summary of fluorochromes used in the assay. (c) Gating strategy applied to cells during sorting. (d) Analysis of FRET response in live versus apoptotic cell populations. Image Credit: Fluidic Sciences and Sphere Bio
Conclusions
- The platform reliably detects both localized (antibody-antigen binding) and dispersed (IgG secretion, FRET signal) fluorescence.
- When combined with fluorescently stained cells, it accurately distinguishes between different cell types based on specific staining.
- The platform demonstrated strong sorting accuracy and effective enrichment of target populations following sequential gating.
- Cyto-Mine® Chroma is a dependable solution for multiplexing assays in both CLD (productivity and viability) and AbD (productivity and antigen specificity).
Acknowledgements
Produced from materials originally authored by Maryam Ahmadi, Elena Shvets, Frank Gesellchen, Jitender Bisht, Joshua Stewart, Manju Namdeo, Marco Stefano Torrisi, Sophie Davies, and Richard Hammond at Fluidic Sciences and Sphere Bio, Granta Centre, Granta Park, Gt Abington, Cambridgeshire, CB21 6AL, UK.
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Oct 25, 2025