Last month a U.S. Food and Drug Administration (FDA) advisory panel had voted 11 to 0 approving a shingles vaccine for its use in adults aged 50 and over. The panel unanimously accepted the safety and efficacy of the vaccine and thus GlaxoSmithKline’s Shingrix.
This new vaccine since then has received the FDA approval this Monday. A federal committee of experts that advises the Centre of Disease Prevention and Control (CDC) yesterday (25th of October 2017), recommends the use of Shingrix for all persons over 50 years of age.
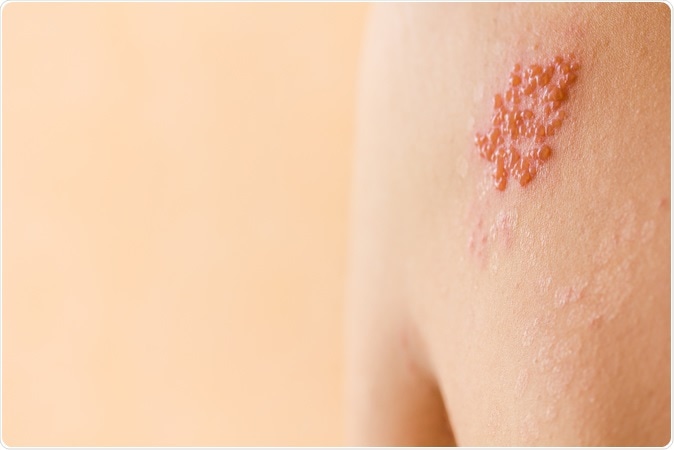
Shingles / herpes zoster. Image Credit: SneSivan / Shutterstock
The Advisory Committee on Immunization Practices advises the CDC on current practices. The committee said that adults who have already received the earlier approved vaccine Zostavax (a shingles vaccine made by Merck) are to be vaccinated again with this new vaccine Shingrix. Shingrix, the committee said, is to be preferred over Zostavax. Until now the only vaccine against herpes zoster was Zostavax that was approved in 2006 for persons over the age of 60 years.
The committee has found that Zostavax is 51 percent effective in preventing the herpes zoster or shingles and 67 percent effective against postherpetic neuralgia whereas Shingrix is 97 percent effective in preventing shingles between ages 50 and 69 years. Shingrix is 91 percent effective in preventing shingles in those over 70 years. Shingrix can prevent postherpetic neuralgia in 91 percent cases over 50 years. These results have been studied in over 38,000 participants making the results reliable. With time Zostavax loses its efficacy. Within one year of vaccination the efficacy of Zostavax is lost by 15 to 25 percent. Within 9 years the protection is lost. Shingrix till date has been studied for up to four years only and it has been seen that its efficacy remains 85 percent for up to four years post vaccination.
According to Dr. Kathleen Dooling, a medical officer in the CDC’s division of viral disease, the final recommendation is expected to be released early next year. Dr. Dooling said, “The Shingrix vaccine has the potential to prevent tens of thousands of cases of shingles and its complications.”
Shingles and its Vaccines
Shingles or herpes zoster is actually a reactivation of the varicella-zoster virus that had caused a chicken pox infection in the person previously. The varicella zoster virus usually remains dormant in a person’s body and in times of weakened immunity such as in old age or during disease, the virus resurfaces as the painful blisters.
The blisters usually cluster in one place on one side of the body leading to burning, tingling or numbing pain. This gets dry and forms crusts over 2 to 3 weeks. The rash affects one side of the torso or may appear over face, forehead, eyes, mouth, and ears on one side. It typically follows distribution and branches of a nerve. Facial shingles is called Ramsay Hunt Syndrome. Sometimes the nerve pain may persist in 10 to 18 percent individuals. This is called Post herpetic neuralgia. When shingles affects the eyes or ears, it can leave a person blind or deaf. According to the CDC, there are approximately 1 million new cases of shingles each year in the US. 20 million people over the age of 60 have received the Zostavax vaccine.
There are essentially two types of vaccines against viral infections;
- Live attenuated vaccine wherein weaker version of the virus is introduced in the body with the vaccine dose to teach the body’s immune system to fight the virus. This way when the virus actually is encountered, the body remembers and fights the infection successfully.
- Subunit vaccine where only a part of the dead virus is introduced into the body. This subunit is an essential part of the virus that the body must recognize. This way too the body learns how to fight the virus when it really encounters it.
Zostavax is a live attenuated vaccine whereas Shingrix is a non-live, subunit vaccine. Because a whole weakened virus is not introduced with the vaccine the risk of getting side effects and adverse events with the vaccine is lower with Shingrix. Zostavax is given as a single shot. For Shingrix the recommended dosage is one shot each given at two months interval.
GSK has assured that the new vaccine would be available on the shelves soon. It is expected to cost about $280. Zostavax costs $223.