Feb 6 2018
Researchers at Okayama University report in Scientific Reports a promising method for delivering viral DNA, able to eliminate cancerous cells, to a tumor. The approach, involving encapsulation of the DNA in liposomes, has the potential to enable intravenous delivery of virus-based antitumor agents.
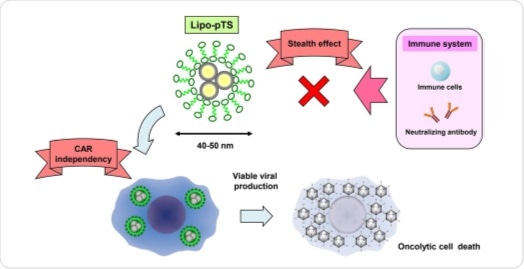
Viral DNA encapsulated in a liposome can be delivered to a tumor site, resulting in oncolytic cell death, without interference of the immune system.
The principle behind oncolytic virotherapy, an emerging cancer treatment strategy, is to introduce viruses in tumors to infect and kill cancer cells. The targeted delivery of oncolytic viruses to a tumor site is, however, a bottleneck in the development of the therapy — normally, non-specific administering of such viruses leads to their neutralization by the immune system. Inspired by the potential of using nanoparticles for drug-delivery, Professor Toshiyoshi Fujiwara and Associate Professor Hiroshi Tazawa, Assistant Professor Shinji Kuroda from Okayama University have now shown that encapsulating the DNA of a particular type of virus in nano-sized vesicles with a lipid-bilayer shell (so-called liposomes) does not trigger the immune system, while still inhibiting tumor growth.
Liposomes as drug or ‘agent’ carriers have the advantage of being stable in the bloodstream and can therefore be easily transported. The size of the agent-liposome unit is crucial, however: particles that are too large are usually captured by the reticuloendothelial system (a set of cells forming part of the immune system), whereas too-small particles end up in urine. Professor Fujiwara and colleagues realized that the virus they wanted to use (of a type known as adenovirus, with a diameter of 90 to 100 nm) would result in a too-largeagent-liposome cluster. They therefore encapsulated only the plasmid DNA of the virus — DNA in non-chromosomal form, but still able to replicate.
The scientists first confirmed that their clusters (abbreviated ‘Lipo-pTS’) display proper cytotoxic activity. In both in vitro and in vivo settings, exposure of cancer cells to Lipo-pTS indeed led to cancer cell death from the action of the viral DNA. Then, the research team found that Lipo-pTS has a ‘stealth effect’ on the immune system: the presence or absence of antibodies to the adenovirus providing the DNA used did not influence cytotoxic function. (Previous experiments with the full adenovirus showed that such antibodies, usually present in adult humans as it is the virus causing the common cold, suppress cytotoxic activity.) Importantly, this implies that systemic delivery of Lipo-pTS, i.e. by oral intake or intravenous injection, is possible.
Professor Fujiwara and colleagues point out that, although their study has limitations — they did not perform animal experiments with systemic administration, for example — they believe that it “shows the promising potential of liposome-encapsulated oncolytic adenovirus … for cancer therapy” and they are hopeful that it “will serve as a foundation for development of systemically-deliverable oncolytic viral agents”.
Source: http://www.okayama-u.ac.jp/