Researchers in France have conducted a study demonstrating the sensitivity of a rapid flow cytometry assay that could be used to screen for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
The assay detects the upregulation of whole blood monocyte CD169, which has been reported as a marker of viral infections.
This rapid assay was more sensitive than the currently used method and less likely to produce false-negative results, especially among early and asymptomatic patients.
The researchers say the diagnostic accuracy, easy finger-prick sampling, and rapid results (15-30 minutes) point to whole blood monocyte CD169 upregulation as a potential new biomarker for COVID-19 screening.
Screening for monocyte CD169 upregulation could decrease the pressure health systems are currently under, reduce overall costs, and make sample collection easier for a significant number of people, says the team.
The study was conducted by researchers from Assistance Publique – Hôpitaux de Marseille (APMH); IHU Méditerranée Infection, Marseille, Aix-Marseille University and Beckman Coulter, Marseille.
The findings can be accessed on the server medRxiv* while the article undergoes peer review.
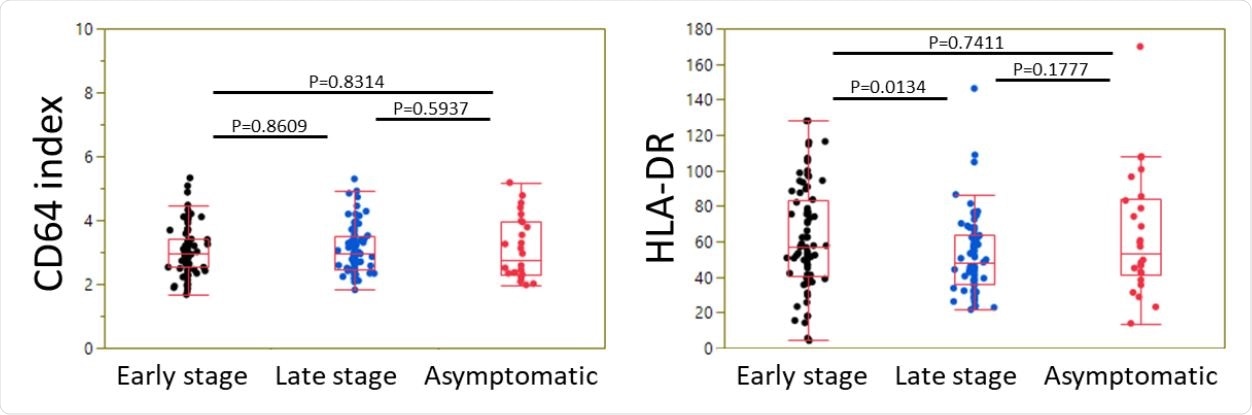
Expression of neutrophil CD64 and monocyte HLA-DR in CoVID-19 patients as a function of disease stage. Box plots summarizing the level of CD64 index (ratio of neutrophil vs lymphocyte signals) (A) and HLA-DR signal on monocytes (B) in CoVID-19 patients at an early stage, at a late stage, or asymptomatic, are shown. Box-and-whisker plots come from the first to the third quartile and are cut by the median; segments at the end are extreme values.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
COVID-19 has spread at an unprecedented rate
Since the COVID-19 outbreak began in Wuhan, China, late last year, the causative agent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread at an unprecedented rate, infecting more than 43.6 million people and killing more than 1.1 million.
Many countries have introduced mass diagnostic testing intending to selectively isolate infected individuals.
“Ideally, this should allow any person to receive a prompt and straightforward answer at the time of symptom onset or contact tracing,” say Moïse Michel (APMH) and colleagues.
The current method of detection
Currently, the method used is the reverse transcriptase-polymerase chain reaction (RT-PCR) test, which detects viral RNA in a nasopharyngeal sample.
However, as well as being a relatively expensive test, the sensitivity of RT-PCR is not optimal, mainly due to the delay between exposure to infection and colonization of the upper ear, nose, and throat area.
Furthermore, although RT-PCR is a fairly fast technique (1 to a few hours), the strain placed on laboratories is significantly delaying sample processing and the delivery of results. The deep nasal swabbing involved is also uncomfortable for the patient and potentially dangerous for the person taking the sample.
Markers of leukocyte activation could help
“In this context, harnessing immune markers of leukocyte activation is a promising tool,” say the researchers. “Indeed, leukocytes detect and rapidly respond to infection with secreted and surface activation molecules.”
CD169 upregulation has been detected in patients with many viruses, including HIV, Ebola, Dengue, and Zika, and mass cytometry studies have now identified CD169 as a relevant biomarker for COVID-19.
Testing monocyte CD169 upregulation as a biomarker of infection
Now, Michel and the team have tested the COVID-19 diagnostic efficacy of a rapid and affordable flow cytometry rapid assay they developed to measure whole blood monocyte CD169 upregulation.
They tested the assay in a large cohort (n=177) of patients with RT-PCR-confirmed SARS-CoV-2 infection. Whole blood CD169 testing was carried out in parallel with RT-PCR screening for SARS-CoV-2.
The cohort was divided into three groups: 80 patients with early-stage infection (≤14 days after symptom onset; group 1), 71 with late-stage infection (≥15 days; group 2), and 26 with asymptomatic infection (group 3).
The CD169 assay was more sensitive than RT-PCR
Whole blood monocyte CD169 evaluation demonstrated greater sensitivity than RT-PCR, particularly among early-stage, asymptomatic patients.
In group 1 (early stage; n=80), the CD169 index was higher than the established 3.5 thresholds in 80% (64) of patients, while RT-PCR detected the virus in 65% (52) of patients.
In group 3 (asymptomatic infection; n=26), significant CD169 upregulation was detected in 46% (12) of patients at levels similar to those observed in group 1, while RT-PCR detected the virus in 35% (9) of patients.
The CD169 assay demonstrated a 98% sensitivity in early-stage disease and a 100% sensitivity in asymptomatic infection. However, the assay did not demonstrate high specificity in late-stage disease, suggesting that CD169 expression decreases once the virus has cleared.
A potential screening strategy
The researchers say the findings show that a testing strategy leveraging monocyte CD169 upregulation as a triage test could be designed.
“Screening for monocyte CD169 upregulation could alleviate the load on specialized RT-PCR services and reduce overall costs while making the sample collection step easier for the greatest number of people,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Michel M, et al. An ultra-sensitive, ultra-fast whole blood monocyte CD169 assay for COVID-19 screening. medRxiv, 2020. doi: https://doi.org/10.1101/2020.10.22.20215749, https://www.medrxiv.org/content/10.1101/2020.10.22.20215749v1
- Peer reviewed and published scientific report.
Michel, Moïse, Fabrice Malergue, Inès Ait Belkacem, Pénélope Bourgoin, Pierre-Emmanuel Morange, Isabelle Arnoux, Tewfik Miloud, et al. 2022. “A Rapid, Easy, and Scalable Whole Blood Monocyte CD169 Assay for Outpatient Screening during SARS-CoV-2 Outbreak, and Potentially Other Emerging Disease Outbreaks.” SAGE Open Medicine 10 (January): 205031212211154. https://doi.org/10.1177/20503121221115483. https://journals.sagepub.com/doi/10.1177/20503121221115483.