Researchers in the United States have conducted a study demonstrating the real-world effectiveness of the recently approved Moderna and Pfizer/BioNtech vaccines at protecting against infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease 2019 (COVID-19).
The team – from Nference in Cambridge, Massachusetts and the Mayo Clinic in Rochester, Minnesota –says the findings are on par with the results reported in large phase 3 randomized clinical trials.
The results showed that the vaccines are effective at both preventing infection and reducing the severity of COVID-19.
Venky Soundararajan and colleagues also demonstrate that vaccination effectively protects individuals at the highest risk of becoming infected with SARS-CoV-2 and experiencing severe disease.
“Building upon the previous randomized trials of these vaccines, this study demonstrates their real-world effectiveness in reducing the rates of SARS-CoV-2 infection and COVID-19 severity among individuals at the highest risk for infection,” write the researchers.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The two vaccines are being rolled out across the United States
In 2020, large phase 3 clinical trials demonstrated that the Pfizer/BioNTech BNT162b2 vaccine is 95% effective at preventing symptomatic COVID-19 and that the Moderna mRNA1273 vaccine is 94.1% effective.
Both of these vaccines are now being rolled-out across the Unites States, with priority given to those at high risk of infection or severe disease.
However, “while these groups were not excluded from the phase 3 trials, vaccine efficacy has not been specifically demonstrated among them,” says Soundararajan and colleagues.
“It is thus critical to analyze outcomes of vaccinated patients to date to determine whether these vaccines are indeed effective in especially high-risk individuals.”
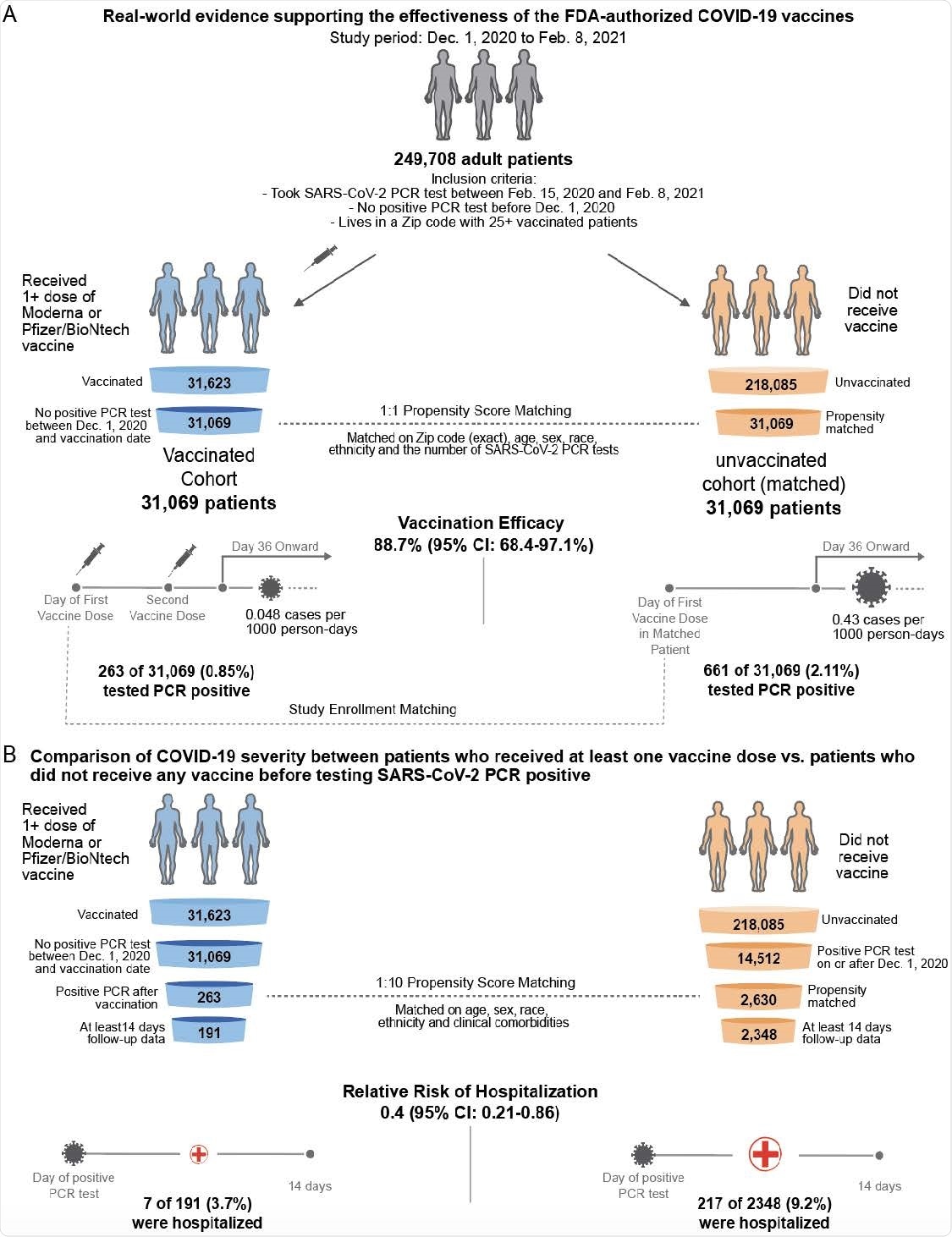
Schematic illustrating the algorithms for participant selection and outcome assessment. (A) Design of study to compare SARS-CoV-2 infection rates in patients receiving COVID-19 vaccination compared to 1-to-1 propensity-matched unvaccinated patients (n = 31,069 per group). For each group, incidence rates were calculated to assess the efficacy of vaccination in preventing SARS-CoV-2 infection, as defined by a positive PCR test, with onset at least 36 days after the first dose or the date of study enrollment. Several other time windows were also evaluated for vaccine efficacy. (B) Design of study to compare COVID-19 disease severity in patients who were vaccinated prior to diagnosis with COVID-19 and had at least 14 days of follow-up after diagnosis (n = 191) versus 1-to-10 propensity-matched unvaccinated patients with at least 14 days of follow-up (n = 2,348). Severity outcomes (hospitalization, ICU admission, and mortality) were assessed within 14 days of PCR diagnosis.
What did the current study involve?
Now, the team has conducted a preliminary assessment of real-world vaccination outcomes in 62,138 individuals within the Mayo Clinic health system (including Arizona, Florida, Minnesota, Wisconsin) between 1st December 2020 and 8th February 2021.
The researchers assessed SARS-CoV-2 positivity and COVID-19 severity among 31,069 individuals who received at least one dose of either the Pfizer/BioNTech BNT162b2 or the Moderna mRNA-1273 vaccine.
“One challenge inherent to such real-world analyses is the lack of a built-in placebo arm, which is essential to establish the expected infection rate during the study period and thereby to assess vaccine efficacy,” writes Soundararajan and colleagues.
To address this shortcoming, the researchers used 1-to-1 propensity score matching to generate a cohort of 31,069 individuals who did not receive a vaccine by the end of the study period.
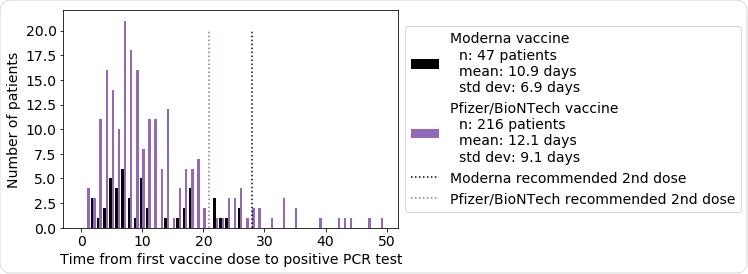
Distribution of the time from first vaccine dose to first positive PCR test, for the patients with at least one positive PCR test following vaccination. Patient counts for mRNA-1273 (Moderna vaccine) are shown in black, and patient counts for BNT162b2 (Pfizer/BioNTech vaccine) are shown in purple. For mRNA-1273, the mean time to positive PCR test following the first dose is 10.9 days (standard deviation: 6.9 days), and for BNT162b2, the mean time to positive PCR test following the first dose is 12.1 days (standard deviation: 9.1 days). Dotted lines indicate the recommended time for the second vaccine dose for mRNA-1273 (28 days) and BNT162b2 (21 days).
Vaccine efficacy reached 88.7%
Starting 36 days following study enrollment, the incidence of SARS-CoV-2 positivity among individuals who received two vaccines was 0.048 cases per 1000 person-days, compared with 0.43 cases per 1,000 days among unvaccinated individuals.
This corresponds to a vaccine efficacy of 88.7%, which the team says is in line with the previously reported efficacies.
Even in the first seven days following enrollment, the incidence of SARS-CoV-2 positivity was significantly lower among vaccinated individuals than among unvaccinated individuals (0.48 versus 1.0 case per 1,000 days), corresponding to an efficacy of 53.6%.
Efficacy then increased over subsequent weeks and reached its maximum during the sixth week after study enrollment.
How did disease severity compare between the two groups?
Among the vaccinated individuals who tested positive for SARS-CoV-2, the 14-day hospitalization rate was significantly lower than among the unvaccinated individuals who tested positive, at 3.7% versus 9.2%.
On the other hand, ICU admission rates were similar between the two cohorts, at 1.0% versus 1.3%, as were 14-day mortality rates, at 0.0% versus 0.085%.
However, the team says it is worth noting that none of the vaccinated patients who developed COVID-19 have died, including 59 individuals who were followed up for at least 28 days.
“A strong real-world effect” of vaccination
“Our data demonstrate a strong real-world effect of COVID-19 vaccination on par with the results reported in each randomized trial,” writes Soundararajan and colleagues.
“We emphasize that COVID-19 vaccines should be administered as broadly and rapidly as possible to the public and that the real-world efficacy of these vaccines should be continuously monitored as we move beyond Phase 1a of the distribution process,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Soundararajan V, et al. FDA-authorized COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.02.15.21251623, https://www.medrxiv.org/content/10.1101/2021.02.15.21251623v1
- Peer reviewed and published scientific report.
Pawlowski, Colin, Patrick Lenehan, Arjun Puranik, Vineet Agarwal, A.J. Venkatakrishnan, Michiel J.M. Niesen, John C. O’Horo, et al. 2021. “FDA-Authorized MRNA COVID-19 Vaccines Are Effective per Real-World Evidence Synthesized across a Multi-State Health System.” Med 2 (8): 979-992.e8. https://doi.org/10.1016/j.medj.2021.06.007. https://www.cell.com/med/fulltext/S2666-6340(21)00238-5?.