Over the last two decades, three major outbreaks of highly pathogenic coronaviruses have occurred. The third is the ongoing coronavirus disease 2019 (COVID-19) pandemic that has claimed well over 2.46 million human lives so far, in a little over a year from its onset. Without any targeted, safe and effective antivirals to prevent or treat the infection by the causative pathogen, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), population immunity via mass vaccination seems to be the only way out – as complex and expensive as the process is likely to be.
This includes existing SARS-related coronaviruses (SARSr-CoVs) in humans, as well as those that are now circulating in animals.
The first evidence that this could be so came from the observation that SARS-CoV caused the production of cross-neutralizing antibodies against many betacoronaviruses (betaCoVs). This proof-of-concept drove the search for a vaccine that would induce neutralizing antibodies against multiple group 2b Sarbecoviruses.
Cross-neutralizing antibodies
Cross-neutralizing antibodies always target the viral receptor-binding domain (RBD) via a specific epitope. The RBD can be rendered more immunogenic by using a multimeric form. One way to achieve this is by using nanoparticles to mount arrays of RBD proteins, creating a virus-like particle (VLP).
Vaccines have been shown to successfully induce cross-neutralizing antibodies against pseudoviruses expressing CoV antigens in mouse studies. The current study describes a non-human primate (NHP) study that explores the cross-neutralizing ability of a SARS-CoV-2 vaccine based on multimeric SARS-CoV-2 RBD-bearing nanoparticles.
RBD-conjugated nanoparticle vaccine
The RBD-conjugated nanoparticle vaccine comprises 24 RBD protomers on a sortase-ferritin platform for the sake of versatility. This bound not only to the human host cell receptor, the angiotensin-converting enzyme 2 (ACE2), which is thought to be the SARS-CoV-2 entry receptor, but also to potent anti-RBD neutralizing antibodies. These include DH1041, DH1042, DH1043, DH1044, and DH1045.
All these antibodies bind to epitopes within the receptor-binding motif, within the RBD. However, antibodies that bound to epitopes outside the RBD were not able to bind the RBD-bearing nanoparticle. In contrast, it did show binding to the cross-neutralizing antibody DH1047.
This vaccine was assessed by a three-dose regimen, administered at four-week intervals, in a non-human primate (NHP) study. The vaccine was found to result in high plasma levels of antibodies to the SARS-CoV-2 RBD and to the stabilized spike protein.
The antibodies completely blocked the ACE2 binding site on the spike protein after two doses of vaccine and partially blocked the binding of the RBD antibody DH104.
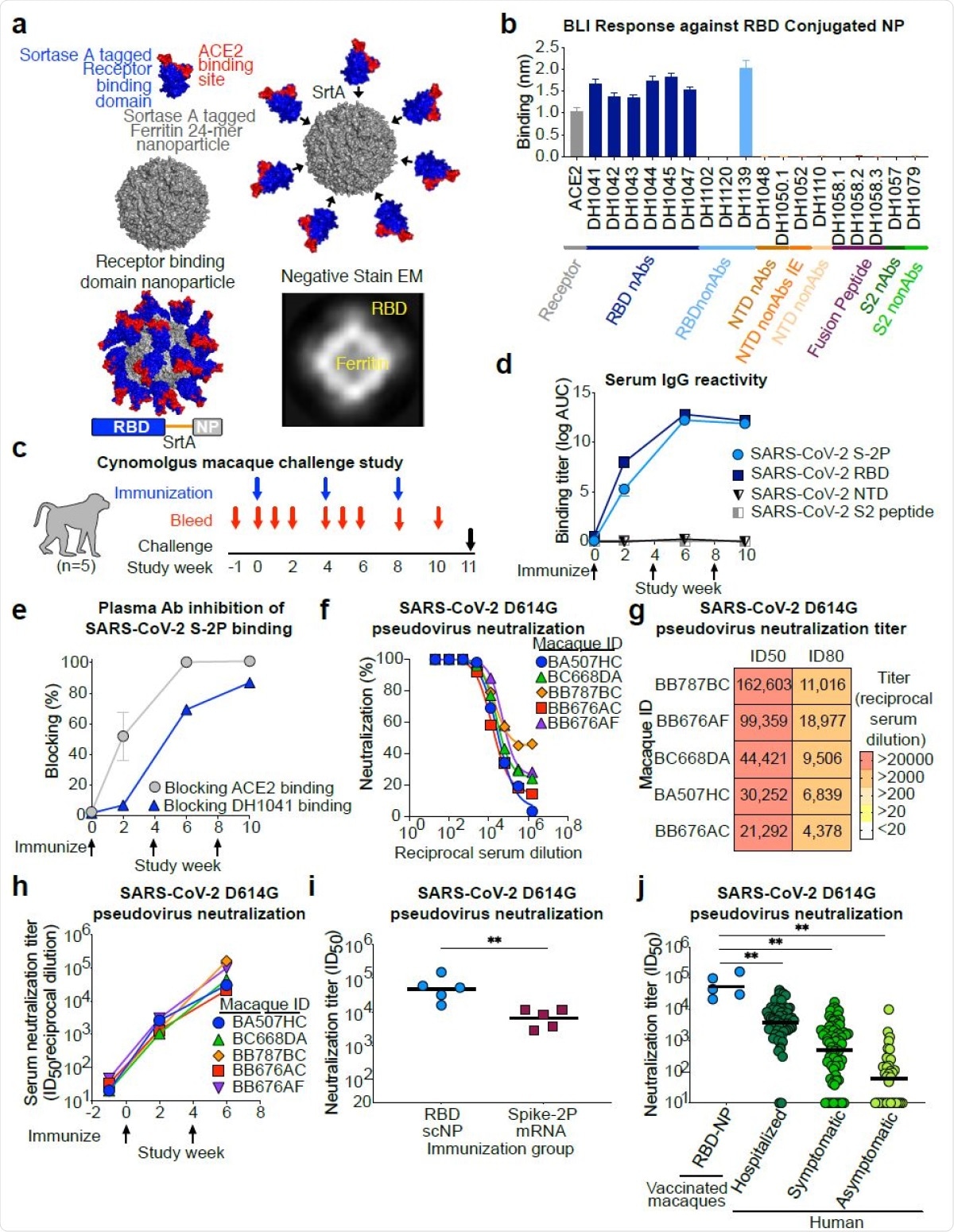
SARS-CoV-2 receptor binding domain (RBD) sortase conjugated nanoparticles (scNPs) elicits extremely high titers of SARS-CoV-2 pseudovirus neutralizing antibodies. a. SARS-CoV-2 RBD nanoparticles were constructed by expressing RBD with a C-terminal sortase A donor sequence (blue and red) and a Helicobacter pylori ferritin nanoparticle with N737 terminal sortase A acceptor sequences (gray) on each subunit (top left). The RBD is shown in blue with the ACE2 binding site in red. The RBD was conjugated to nanoparticles by a sortase A (SrtA) enzyme conjugation reaction (top right). The resultant nanoparticle is modeled on the bottom left. Nine amino acid sortase linker is shown in orange. Two dimensional class averages of negative stain electron microscopy images of actual RBD nanoparticles are shown on the bottom right. b. Antigenicity of RBD nanoparticles determined by biolayer interferometry against a panel of SARS-CoV-2 antibodies and the ACE2 receptor. Antibodies are color-coded based on epitope and function. N-terminal domain (NTD), nonAbs IE, infection enhancing non-neutralizing antibody; nAb, neutralizing antibody; nonAb, non-neutralizing antibody. Mean and standard error from 3 independent experiments are shown. c. Cynomolgus macaque challenge study scheme. Blue arrows indicate 748 RBD-NP immunization timepoints. Intranasal/intratracheal SARS-CoV-2 challenge is indicated at week 10. d. Macaque serum IgG binding determined by ELISA to recombinant SARS-CoV-2 stabilized Spike ectodomain (S-2P), RBD, NTD, and Fusion peptide (FP). Binding titer is shown as area752under-the curve of the log10-transformed curve. Arrows indicate immunization timepoints. e. Plasma antibody blocking of SARS-CoV-2 S-2P binding to ACE2-Fc and RBD neutralizing antibody DH1041. Group mean and standard error are shown. f. Dose-dependent serum neutralization of SARS-COV-2 pseudotyped virus infection of ACE2- expressing 293T cells. Serum was collected after two immunizations. The SARS-CoV-2 pseudovirus spike has an aspartic acid to glycine change at position 614 (D614G). Each curve represents a single macaque. g. Heat map of serum neutralization ID50 and ID80 titers for SARS-COV-2 D614G pseudotyped virus after two immunizations. h. SARS-COV-2 D614G pseudotyped virus serum neutralization kinetics. Each curve represents a single macaque. i. Comparison of serum neutralization ID50 titers from cynomolgus macaques immunized with recombinant protein RBD nanoparticles (blue) or nucleoside-modified mRNA-LNP expressing S- 2P (burgundy) (**P<0.01, Two-tailed Exact Wilcoxon test n = 5). j. Comparison of serum neutralization titers obtained from RBD-scNP-vaccinated macaques (blue) and SARS-CoV-2 infected humans (shades of green). Human samples were stratified based on disease severity as asymptomatic (N=34), symptomatic (n=71), and hospitalized (N=60) (**P<0.01, Two-tailed Wilcoxon test n = 5).
Competitive with the Moderna/Pfizer vaccine for neutralizing antibody titer
When tested against the currently dominant D614G strain of SARS-CoV-2, the RBD-conjugated nanoparticle vaccine induced higher neutralizing antibody titers than another vaccine similar to the Moderna and Pfizer lipid-encapsulated nucleoside-modified mRNA (mRNA-LNP) vaccines that are now being used in the vaccination campaigns against COVID-19.
The measure of antibody titer used here showed an eight-fold increase with the former compared to the latter. The antibody response was also higher with the RBD-nanoparticle vaccine than with natural infection of all grades of severity.
Unaffected by emerging variants
It also showed potent neutralizing activity against the new SARS-CoV-2 variant B.1.1.7, which is rapidly spreading worldwide. This is not only more infective but may be resistant to many RBD-targeting antibodies, as well as more virulent.
While changes in binding affinity of anti-RBD antibody DH1041 to the ACE2 receptor and to the spike protein were observed with different mutations, such as those acquired during mink infection, or those found in the South African or Brazil or UK strains, the cross-neutralizing antibody DH1047 showed unchanged binding to the SARS-CoV-2.
“RBD-scNP (RBD sortase A conjugated nanoparticle) and mRNA-LNP-induced RBD binding antibodies were not sensitive to spike mutations present in neutralization-resistant UK, South Africa or Brazil SARS-CoV-2 variants.”
SARS-CoV-2 spike induces cross-neutralizing antibodies to pre-emergent betaCoVs
SARSr-CoVs still pose a danger of future pandemics to human beings. The researchers, therefore, explored the ability of this vaccine to neutralize other viruses. Similar to the LNP-mRNA vaccines based on the prefusion stabilized spike or the RBD, the RBD-scNP also elicited potent cross-neutralizing antibodies against SARS-CoV and SARSr-bat CoVs (batCoV-WIV-1, and batCoV-SHC014).
The neutralization was most potent against SARS-CoV-2, however. The highest neutralizing antibody titers were observed with RBD-scNP and the least with the RBD-expressing LNP-mRNA vaccine. The high titers may indicate that durable immunity is achieved.
The RBD-scNP vaccine showed cross-neutralizing activity against batCoV-RaTG13 and pangolin CoV GXP4L spike antigens, in addition to SARS-CoV and SARS-CoV-2. Notably, sera obtained following vaccination with this formulation failed to neutralize the seasonal human CoVs or MERS-CoV, probably because of the difference in RBD among these CoVs, which belong to different groups.
The similarity between the RBD-scNP and DH1047 in terms of cross-neutralizing profile shows that not only do antibodies induced by the former bind near the epitope bound by the latter, but they are not specific to SARS-CoV-2 RBD. In fact, they also block batCoV-SHC01.
Notably, only a third of COVID-19 patients produce antibodies that block DH1047, indicating it is a sub-immunodominant epitope. As such, the RBD-scNP vaccine targets this epitope rather than the immunodominant ACE2 blocking epitope.
Protection against productive infection
The RBD-scNP vaccine was also protective for vaccinated monkeys when challenged with the SARS-CoV-2 virus via the respiratory tract. In all but one of the vaccinated macaques, “RBD-scNP-induced immunity prevented virus replication, and likely provided sterilizing immunity, in the upper and lower respiratory tract.”
What are the implications?
The RBD-scNP platform induced the highest cross-neutralizing antibody titer for group 2b CoVs, and as such, may serve as the basis for a reasonably effective initial broadly neutralizing vaccine against this group – both now, and in the future, if the further zoonotic transmission should occur.
The study also showed that the use of both RBD-scNP and the LNP-spike mRNA vaccines, the latter resembling those which have been recently rolled out, is capable of inducing cross-neutralizing antibodies to the dominant D614G variant and the newer variants of SARS-CoV-2, but at lower titers.
The findings indicate the ability of the SARS-CoV-2 Spike to be included in an RBD-scNP or LNP-mRNA formulation to induce cross-neutralizing antibodies against several SARSr-CoVs. Thus, even the currently used vaccines are likely to prevent future pandemics if immunization is successfully achieved.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Saunders, K. O. et al. (2021). SARS-CoV-2 vaccination induces neutralizing antibodies against pandemic and pre-emergent SARS-related coronaviruses in monkeys. bioRxiv preprint. doi: https://doi.org/10.1101/2021.02.17.431492. https://www.biorxiv.org/content/10.1101/2021.02.17.431492v1
- Peer reviewed and published scientific report.
Saunders, Kevin O., Esther Lee, Robert Parks, David R. Martinez, Dapeng Li, Haiyan Chen, Robert J. Edwards, et al. 2021. “Neutralizing Antibody Vaccine for Pandemic and Pre-Emergent Coronaviruses.” Nature, May, 1–9. https://doi.org/10.1038/s41586-021-03594-0. https://www.nature.com/articles/s41586-021-03594-0.