The search for effective inhibitors with therapeutic potential against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the shattering pandemic of coronavirus disease 2019 (COVID-19), has thrown up some surprising results.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
MARCH8 inhibits envelope protein expression
The membrane-associated RING-CH (MARCH) 8 protein is one of eleven similar proteins that form one category of RING-finger E3 ubiquitin ligases. It has two transmembrane domains linked by a short ectodomain. It reduces the expression of many host transmembrane proteins, such as the strategically important major histocompatibility complex II (MHC-II), which presents antigens correctly to immune responder cells for immune activation.
Recently, the researchers in the current paper had shown that MARCH8-mediated inhibition of ubiquitination has the ability to impair the expression of envelope glycoproteins on HIV-1, and thus reduce the infectivity of the virions released from cells expressing MARCH8.
Similar effects were observed with the vesicular stomatitis virus G-glycoprotein (VSV-G), which bears no resemblance to the HIV-1 envelope protein. This inhibition occurs through a different mechanism, mediated by the tyrosine motif.
The study therefore aimed to explore the hypothesis that MARCH8 may inhibit a variety of envelope proteins belonging to different viruses, using multiple infectivity assays.
MARCH8 inhibits pseudovirus infection
The antiviral spectrum of MARCH8 was explored using pseudoviruses expressing several different viral envelope glycoproteins. This included the rhabdovirus rabies virus glycoprotein, rabies-G, the arenavirus lymphocytic choriomeningitis virus (LCMV) envelope glycoprotein, severe acute respiratory syndrome-associated coronavirus (SARS-CoV) and SARS-CoV-2 spike (S) proteins, and the envelopes of two alphaviruses, Chikungunya virus (CHIKV) and the Ross River virus (RRV) envelope.
The findings show that all of them were inhibited in a dose-dependent manner, with the LCMV-GP and the CHIKV envelope being especially susceptible even at low concentrations of MARCH8.
MARCH8 targets cytoplasmic lysine residues
Earlier research showed that VSV-G was inhibited by MARCH8 via ubiquitination and degradation of cytoplasmic lysine residues. Similarly, the rabies-G protein, which has three lysine residues in its single transmembrane domain, is also sensitive to MARCH8.
The mutation of these lysines to arginines conferred resistance to MARCH8. The effect was even more drastic with LCMV-GP, which completely lost its susceptibility to MARCH8 when its six lysine residues were switched out for arginine.
Both SARS-CoV and SARS-CoV-2 have single transmembrane domains, with four lysine residues in the cytoplasmic tails, and again, these became less susceptible to MARCH8 when mutated to arginine residues.
Immunofluorescence results validated these findings, showing a lower expression of these mutant envelopes compared to wildtype.
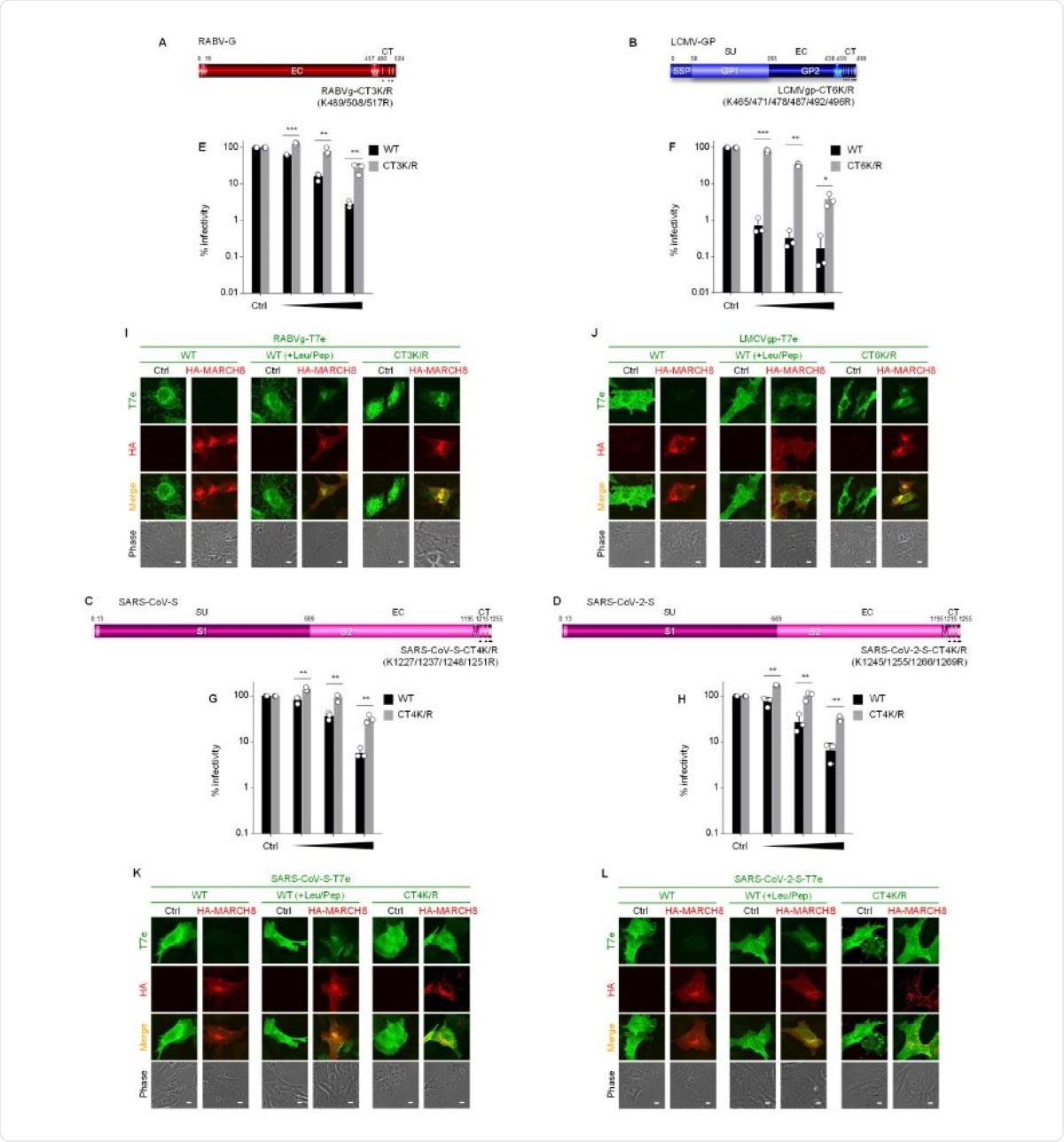
MARCH8 targets lysine residues in the cytoplasmic tail of viral envelopes with single transmembrane domains. (A-D) Schematic gene structures of the lysine mutants of (A) RABV-G (CT3K/R); (B) LCMV-GP (CT-6K/R); (C) SARS-CoV-S (CT-4K/R); and (D) SARS-CoV-2-S (CT-4K/R). SP, signal peptide; EC, extracellular domain; TM, transmembrane domain; CT, cytoplasmic tail; SU, surface subunit. (E-H) MARCH8 resistance is conferred by mutations in cytoplasmic lysine residues of viral envelopes with single transmembrane domains. Infectivity assays were performed as described in Figure 1A except that the maximum amount of MARCH8 plasmid used was 120 ng. Black and gray columns represent the wild-type (WT) and lysine mutants of each viral envelope glycoprotein, respectively. Data from three independent experiments are shown as a percentage of the infectivity of viruses produced in the absence of MARCH8 when the WT protein or its mutant was used (mean ± s.d., n = 3 technical replicates). *p<0.05, **p<0.005, ***p<0.0005, ****p<0.0001 compared with the WT using sing two-tailed unpaired t-tests. ns, not significant. (I-L) Lysine mutants of viral envelopes are resistant to MARCH8-mediated lysosomal degradation. Shown are immunofluorescence-based analyses of the expression of either the T7 epitope (T7e)-tagged WT or K/R mutant of (I) RABV-G; (J) LCMV-GP; (K) SARS-CoV-S; and (L) SARS-CoV-2-S with or without MARCH8 in transfected HOS cells. All WT viral envelopes were rescued from MARCH8-induced degradation in the presence of lysosomal protease inhibitors (+Leu/Pep), as shown in each of the middle panels. Scale bars, 10 μm.
MARCH8 targets cytoplasmic lysine in alphavirus E2 proteins
The researchers found that in the case of the alphaviruses, CHIKV and RRV, substitution of the non-cytoplasmic lysine residues did not produce resistance to MARCH8. However, mutation of the lysine residues on the cytoplasmic lysine residues showed a marked reduction in susceptibility of CHIKV, though the reduction was less drastic with RRV.
These results were also confirmed by immunofluorescence, indicating that the mutation of lysine in the cytoplasmic domains alone was responsible for rescue from MARCH8-mediated ubiquitination and lysosomal breakdown of the envelope proteins.
This was confirmed by immunoprecipitation-cum-Western blot assays of ubiquitination, which was shown to be absent in the lysine-mutants of the rabies and CHIKV envelope proteins.
Tyrosine motif-mediated activity
The LCMV-GP and the CHIKV envelope protein were very sensitive to MARCH8, even after lysine mutation. This was unlike the almost total resistance observed with rabies, SARS-CoV, SARS-CoV-2 and RRV-E3-E2-6K-E1 following substitution of lysine with arginine. This indicates that both ubiquitination and tyrosine motif-dependent downregulation of viral envelope proteins are at work in the former viruses.
MARCH8 was the most inhibitory among all viral envelopes tested, though MARCH1 and MARCH2 have also been reported to have antiviral activity.
Whole-virus experiments show antiviral activity of MARCH8
The researchers were able to repeat the above results with whole rabies viruses in cell culture, where the G protein was not expressed at all on the surface of cells that had MARCH8, but the envelope phosphoprotein P was normally expressed. This confirmed that the antiviral activity was MARCH8-specific.
What are the implications?
The results show that viruses belonging to different families show MARCH8 sensitivity depending on the number of lysine residues in the cytoplasmic tail domains. This depends on the exposure of the lysines to ubiquitin ligase for ubiquitination.
An exception is HIV-1 with two lysines in its Env protein, which remains sensitive to MARCH8 after substituting the lysines with arginine.
The alphaviruses seem to show that not just the number but also the location of the lysine residues affects MARCH8 sensitivity, possibly by impairing access to the latter.
In cells that do not express MARCH8, the lysine-to-arginine mutant forms are more infective than the wildtype viruses by 1.5-5-fold, with SARS-CoV-2 showing the greatest increase. This cannot be explained as evasion of inhibition because the endogenous expression of MARCH8 is very low.
The researchers do not rule out the activity of an unknown E3 ubiquitin ligase in the HEK293T cells used in this study, which might have influenced these results.
There is a clear demarcation in MARCH8 sensitivity between the VSV-G, LCMV-GP, and CHIKV-E3-E2-6K-E1 proteins, and the RABV-G, SARS-CoV-S, SARS-CoV-2-S, and RRV-E3-E2-6K-E1.
In the first group, there is high wildtype sensitivity and resistance in the mutant form, while the second shows dose-dependent MARCH8 sensitivity of the wildtype envelope, but only partial resistance after mutation.
This may indicate that the first group is downregulated via a ubiquitination-dependent pathway, but the second using both this and a tyrosine motif-dependent pathway. The HIV-1 Env protein is an interesting exception, being independent of ubiquitination but dependent on the tyrosine motif.
MARCH8 has been shown very recently to have a still broader spectrum of antiviral activity, including influenza, Ebola, murine leukemia and Nipah virus envelope glycoproteins. It is thus worthy of further research as a host defense mechanism.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Zhang, Y. et al. (2021). MARCH8 targets cytoplasmic lysine residues of various viral envelope glycoproteins. bioRxiv preprint. doi: https://doi.org/10.1101/2021.04.20.440588, https:/www.biorxiv.org/content/10.1101/2021.04.20.440588v1
- Peer reviewed and published scientific report.
Zhang, Yanzhao, Seiya Ozono, Takuya Tada, Minoru Tobiume, Masanori Kameoka, Satoshi Kishigami, Hideaki Fujita, and Kenzo Tokunaga. 2022. “MARCH8 Targets Cytoplasmic Lysine Residues of Various Viral Envelope Glycoproteins.” Edited by Manjula Kalia. Microbiology Spectrum 10 (1). https://doi.org/10.1128/spectrum.00618-21. https://journals.asm.org/doi/10.1128/spectrum.00618-21.