Against the background of the ongoing coronavirus disease 2019 (COVID-19) pandemic, huge disruptions have occurred in healthcare access, social interactions and economic activity. Vaccines have been developed at a highly accelerated pace to end the suffering earlier and at a lower cost than would be achievable by allowing the virus to take its course.
However, the dosage, the immunization schedule, the duration and the breadth of resulting immunity are still matters of study even as over a dozen vaccines are already under emergency use nationwide in many different countries the world over. The logistical difficulties, delay in vaccine supplies, and shortage of manufactured doses have combined to slow the rate of vaccination to the point that it appears unlikely that adequate global coverage will be accomplished within the next two years.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
A recent report on the medRxiv* preprint server suggests that a single dose of the Oxford-AstraZeneca (ChAdOx1 nCoV-19) vaccine could elicit higher titers of neutralizing antibodies, effectively acting as a booster dose, in people who have already been infected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This vaccine does not require ultra-cold storage and transport temperatures, unlike the mRNA vaccines, though it must be stored at fridge temperatures (2-8oC).
Study aim
The Pfizer and Moderna vaccines, developed on a messenger ribonucleic acid (mRNA) platform, have shown the ability to induce impressive protective antibody responses against the virus after a single dose in a previously infected person. Prior infection is associated with the development of immunoglobulin (IgG) antibodies directed against the spike, as well as neutralizing antibody titers equivalent to or even higher than those elicited by two doses of the vaccine. This holds true for both the UK variant B.1.1.7 and the South African variant B.1.351.
This has led to the consideration that a single dose of vaccine is adequate for this group, and a second dose may be, in fact, more likely to induce vaccine-related side effects.
The same has not been reported to any large extent with the Oxford vaccine, which is built on an adenovirus vector platform. Therefore, two doses of the latter are still required in current vaccination protocols, even if the recipient has a history of prior infection.
The current study, therefore, reports the results of a comparison between IgG antibodies to the viral spike antigen, and the neutralizing capacity of these antibodies, towards wildtype SARS-CoV-2 and three variants of concern (VOCs). These comprise the B.1.1.7, B.1.351, and P1 (Brazil) variant.
Details of the study
The comparison cohorts consisted of healthcare workers with and without a history of prior COVID-19. The comparison was made between two doses of the Pfizer mRNA vaccine BNT162b2 and one dose of the Oxford-AstraZeneca vaccine.
Both cohorts that received two doses of the Pfizer vaccine had a median age of 52 and were predominantly female. One cohort had a history of infection; the other was naïve.
Another pair of cohorts included those who received a single dose of the Oxford vaccine after prior natural infection with SARS-CoV-2. One group was less than 11 months from the infection at the date of vaccination, while the other had been infected before this.
Two control groups included unvaccinated previously infected individuals and unvaccinated individuals without evidence of prior infection.
What were the findings?
The study shows high levels of spike-targeting IgG and neutralizing antibodies in all cases, after a single dose of the Oxford-AstraZeneca vaccine, whether the person had been previously infected with the wildtype strain or with the other variants. This also holds true whether the infection occurred within or beyond 11 months prior to the date of vaccination.
The antibody titers and neutralizing activity were similar or even higher than those elicited in those who received two doses of the Pfizer vaccine.
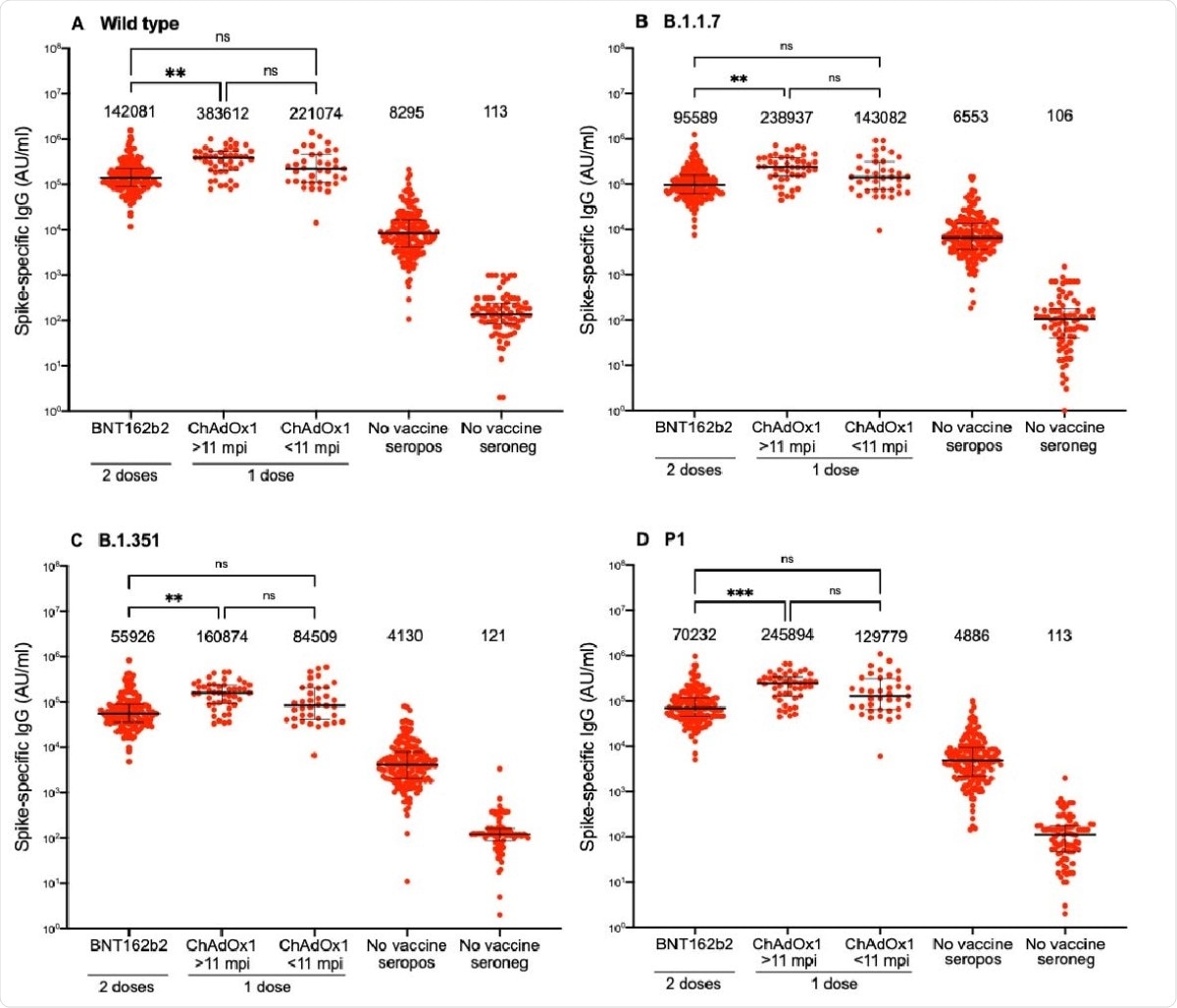
Spike-specific IgG responses against SARS-CoV-2 wild type and variants following two doses BNT162b2 in individuals with or without previous SARS-CoV-2 infection or one dose ChAdOx1 nCoV-19 vaccine in individuals with previous SARS-CoV-2 infection. A) Spike-specific IgG responses against wild type (A), B.1.1.7 (B), B.1.351 (C) and P1 (D). Numbers above each cluster indicate median AU/ml. Lines indicate medians with interquartile ranges. AU; arbitrary units, mpi; months post infection, ns; non-significant, *; p<0.05, **; p<0,01 ***; p<0.001, ****; p<0.0001.
What are the implications?
The researchers conclude that one dose of the Oxford-AstraZeneca vaccine in a previously infected recipient is an effective booster of the specific immune reaction to SARS-CoV-2 when administered 11 or more months after natural infection. The elicited antibody titer and neutralizing activity are comparable to that observed after two doses of the Pfizer vaccine.
This could help stretch available doses to twice the eligible population and thus protect a greater proportion of people against symptomatic and severe disease following SARS-CoV-2 infection.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Havervall, S. et al. (2021). Antibody Responses After a Single Dose of ChAdOx1 nCoV-19 Vaccine in Healthcare Workers Previously Infected with SARS-CoV-2. medRxiv preprint. doi: https://doi.org/10.1101/2021.05.08.21256866, https://www.medrxiv.org/content/10.1101/2021.05.08.21256866v1
- Peer reviewed and published scientific report.
Havervall, Sebastian, Ulrika Marking, Nina Greilert-Norin, Henry Ng, Max Gordon, Ann-Christin Salomonsson, Cecilia Hellström, et al. 2021. “Antibody Responses after a Single Dose of ChAdOx1 NCoV-19 Vaccine in Healthcare Workers Previously Infected with SARS-CoV-2.” EBioMedicine 70 (August): 103523. https://doi.org/10.1016/j.ebiom.2021.103523. https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(21)00316-9/fulltext.