There are now many approved vaccines to combat COVID-19, the disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Most of the vaccines and monoclonal antibodies target the virus spike protein. Over the last few months, several virus variants have emerged that have mutations on the spike protein, raising questions about whether approved vaccines can neutralize them.
Many of the key mutations seen so far have been on the spike protein receptor-binding domain (RBD). Many of these mutations seem to increase the affinity for human angiotensin-converting enzyme 2 (ACE2), thereby increasing transmissibility, whereas others alter the RBD, preventing neutralization by antibodies.
Many antibodies have been identified that target the spike protein. They can be categorized based on their mode of binding and cross-reactivity with other sarbecoviruses. However, many monoclonal and polyclonal antibodies have lost some of their neutralizing potency in the face of these new variants.
In a new study, researchers used an antibody discovery technology to identify a new potently neutralizing antibody with a unique mode of SARS-CoV-2 recognition. They reported their results on the bioRxiv* preprint server.
Identifying antibody
The team used a technology called Linking B Cell receptor to antigen specificity through sequencing (LIBRA-seq), which determines B cell receptor sequences and antigen reactivity at the single-cell level simultaneously. They tested B cells isolated from a convalescent patient about three months after infection.
The team identified a potently neutralizing antibody called 54042-4, which they selected for continued investigation. Using a receptor-blocking assay, the team determined that the antibody inhibits SARS-CoV-2 interaction with ACE2.
A competition assay with three other RBD-directed antibodies revealed that 54042-4 competed for binding with COV2-2130 antibody but not with others. This suggests the 54042-4 antibody targets a portion of the spike RBD that overlaps somewhat with ACE2 binding sites and sites for other neutralizing antibodies.
Cryo-electron microscopy of the structure of the antibody binding fragments to the virus showed that the antibody forms a wide interface with the RBD, with the primary interactions at residues 439–450. The structure also showed that the majority of mutations in the variants of concern would not affect the antibody's binding.
No known antibodies with similar sequences to the new one were found by the team. Although the epitopes of two other antibodies are similar to the new antibody, the other antibodies approach the epitopes at a distinct angle. Although the 54042-4 and 2-7 antibodies arise from the same germline gene, they have different interactions with the virus. Thus, the new antibody is believed to use a distinctive genetic recognition mechanism.
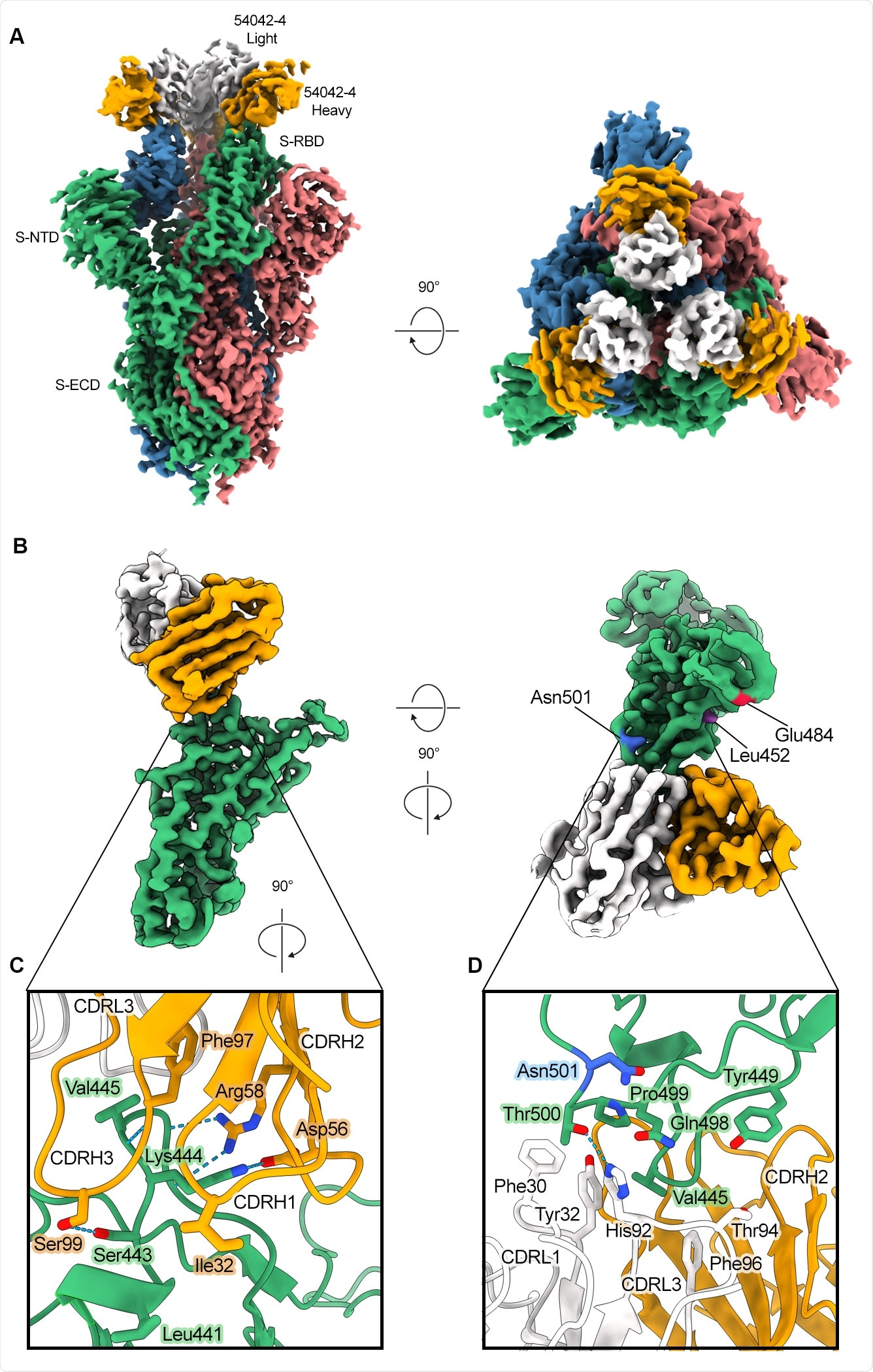
Atomic resolution of 54042-4 binding mode to SARS-CoV-2 S (A) 3D reconstructions of side and top views of Fab 42-4 bound to SARS-CoV-2 spike. (B) Focused refinement maps showing the 54042-4 epitope at the apex of the RBM in the down position (left). Top-down view of the 54042-4 epitope showing heavy and light chain contacts, as well as residues outside of the binding interface that are mutated in circulating VOCs (right).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Potent neutralizing activity
The authors next used a test to identify substitutions that are capable of disrupting the binding of the antibody. They found some substitutions, K444A, V445A, G446A, and P499A, which could affect neutralization by this antibody.
Testing using chimeric SARS-CoV-2 viruses revealed substitutions at Lys444, Gly446, and Gln498 were resistant to neutralization by this antibody. However, substitutions at these residues are only found at low numbers in currently circulating isolates of SARS-CoV-2, and almost all the 54042-4 epitopes are conserved in circulating strains.
The team next tested the binding of antibody 54042-4 to RBD proteins containing substitutions found in the different variants of concern. They found the antibody binding was at a similar level to all the substitutions compared to the original Wuhan strain. The antibody also neutralized the B.1.1.7 and B.1.351 virus strains.
Consequently, the newly discovered antibody exhibits potent neutralizing abilities and is capable of neutralizing almost all of the differences between the currently circulating strains, thus it could complement existing strategies for combating COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Kramer, K. J. et al. (2021) Potent neutralization of SARS-CoV-2 variants of concern by an antibody with a unique genetic signature and structural mode of spike recognition. bioRxiv, https://doi.org/10.1101/2021.05.16.444004, https://www.biorxiv.org/content/10.1101/2021.05.16.444004v1
- Peer reviewed and published scientific report.
Kramer, Kevin J., Nicole V. Johnson, Andrea R. Shiakolas, Naveenchandra Suryadevara, Sivakumar Periasamy, Nagarajan Raju, Jazmean K. Williams, et al. 2021. “Potent Neutralization of SARS-CoV-2 Variants of Concern by an Antibody with an Uncommon Genetic Signature and Structural Mode of Spike Recognition.” Cell Reports 37 (1): 109784. https://doi.org/10.1016/j.celrep.2021.109784. https://www.cell.com/cell-reports/fulltext/S2211-1247(21)01243-2.