Scientists from Germany and Belgium have recently described the immunogenicity of a plant-based virus-like particle vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The vaccine induces robust antibody and T cell responses for at least 6 months. Moreover, the vaccine demonstrates potent neutralizing efficacy against the B.1.1.7, B.1.351, and P1 variants of SARS-CoV-2. The study is currently available on the medRxiv* preprint server.
The COVID-19 outbreak is the biggest pandemic in modern history, with more than 203 million affected people and 4.31 million deaths. Because of the limited availability of specific treatments, management of the COVID-19 pandemic mostly depends on mass vaccination together with non-pharmacological control measures.
The anti-pathogen efficacy of a vaccine primarily depends on its ability to induce neutralizing antibodies. Moreover, the ability to induce cross-reactive antibodies is another vital measure to protect against emerging viral variants. According to available evidence, most of the COVID-19 vaccines are capable of inducing neutralizing antibody titers for at least 8 months.
In the current study, the scientists have described the robustness and durability of immune responses induced by an AS03-adjuvanted, plant-based virus-like particle vaccine containing full-length spike protein of wildtype SARS-CoV-2 as an immunogen.
Study design
The study was conducted on 20 healthy adults who received two doses of 3.75 µg vaccine at an interval of 21 days. The blood samples were collected from the participants 21 days after each immunization and 6 months after the second immunization.
Antibody-mediated immune responses
Immunoglobulin G (IgG)-specific anti-spike binding antibodies were detected in all participants 21 days after the second vaccine dose. The antibodies remained detectable for at least 6 months after the final vaccination. The levels of spike-binding antibodies detected at day 21 post first vaccination were comparable to that observed in COVID-19 recovered patients; however, the levels were significantly lower than that detected at day 21 post-second vaccination.
The neutralizing antibody levels peaked at day 21 after the second vaccination, followed by a gradual decline. However, the levels detected after 6 months of second vaccination were comparable to that in convalescent plasma.
According to the exponential-decay model, the estimated half-life of binding and neutralizing antibodies was around 55 days.
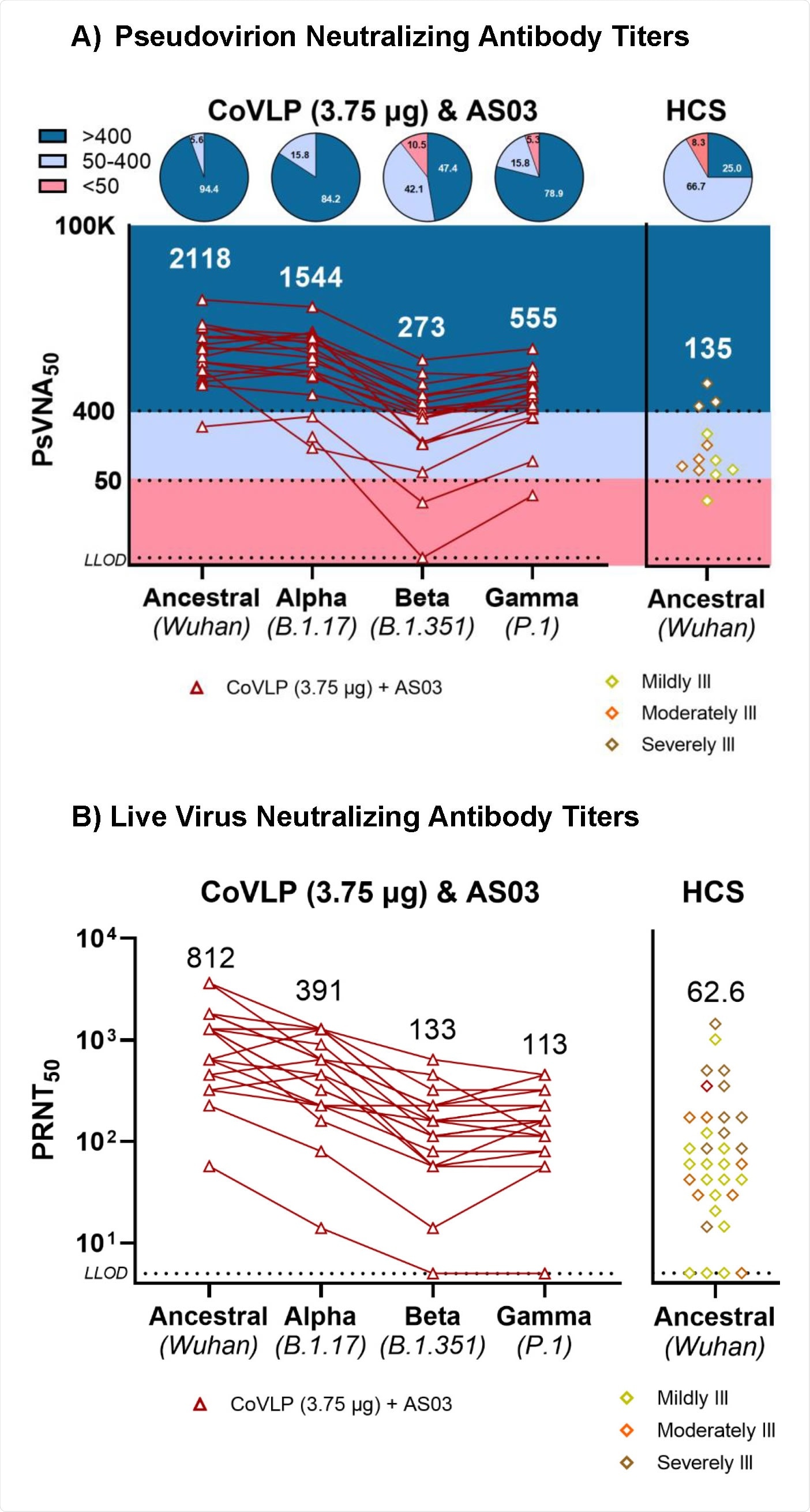
To evaluate cross-reactivity, Day 42 NAbs of subjects vaccinated with 3.75 µg CoVLP adjuvanted with AS03 were quantified in a (A) VSV pseudovirion neutralization assay or (B) live virus neutralization assay with the ancestral Wuhan strain and the Alpha, Beta, and Gamma variants. Convalescent sera or plasma (HCS) samples were collected at least 14 days after a positive diagnosis of COVID-19 (RTPCR) from individuals whose illness was classified as mild, moderate, or severe/critical (Panel A: n=35, Panel B: n=12). Individual values are indicated with red lines; geometric means are indicated above each variant. In panel A, background colors indicate dilution titers; pie charts provide proportions per dilution titers for each variant tested.
Cell-mediated immune response
Interferon γ (IFN-γ)-secreting type 1 T helper cell response was detected in all participants at day 21 post final vaccination. The response remained detectable even after 6 months. A similar trend was also observed for interleukin-4 (IL-4)-secreting type 2 T helper cell response.
The intensity of both IFN-γ and IL-4 responses was highest at day 21 post final vaccination, followed by a gradual decline. Despite the reduction in intensity, the presence of T cell response in almost all participants after 6 months of final vaccination highlights the vaccine's effectiveness in inducing a durable cellular immune response.
Antibody cross-reactivity
Serum samples obtained from vaccinated individuals and COVID-19 recovered patients showed high reactivity to SARS-CoV-2 spike protein. However, the magnitude of reactivity was higher for vaccinated individuals. In addition, significantly higher levels of cross-reactive antibodies against the SARS-CoV spike protein were observed in vaccinated sera compared to that in convalescent sera. Neither significant cross-reactivity to the spike proteins of Middle East respiratory syndrome coronavirus and common cold coronavirus was observed in both vaccinated and convalescent sera.
Regarding SARS-CoV-2 variants of concern (VOCs), all participants exhibited antibody-mediated neutralization of the B.1.1.7, B.1.351, and P1 variants at day 21 post final vaccination. Overall, the highest neutralizing antibody titer was observed against the B.1.1.7 variant, and the lowest titer was observed against the B.1.351 variant.
Taken together, the study highlights the potency of a plant-based virus-like particle vaccine in inducing robust humoral and cellular immune responses after 21 days of final immunization. Although lower in magnitude, the responses remain detectable even after 6 months. Importantly, at day 21 post final vaccination, almost all vaccine recipients have exhibited detectable neutralizing antibodies against some of the SARS-CoV-2 VOCs.
The vaccine is currently under investigation in a large phase 3 clinical trial involving many countries with the active circulation of multiple VOCs.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Gobeil P. 2021. Durability and Cross-Reactivity of Immune Responses Induced by an AS03 Adjuvanted Plant-Based Recombinant Virus-Like Particle Vaccine for COVID-19, medRxiv, https://doi.org/10.1101/2021.08.04.21261507, https://www.medrxiv.org/content/10.1101/2021.08.04.21261507v1
- Peer reviewed and published scientific report.
Gobeil, Philipe, Stéphane Pillet, Iohann Boulay, Nathalie Charland, Aurélien Lorin, Matthew P. Cheng, Donald C. Vinh, et al. 2022. “Durability and Cross-Reactivity of Immune Responses Induced by a Plant-Based Virus-like Particle Vaccine for COVID-19.” Nature Communications 13 (1): 6905. https://doi.org/10.1038/s41467-022-34728-1, https://www.nature.com/articles/s41467-022-34728-1