The spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) over the whole world, causing successive waves of coronavirus disease 2019 (COVID-19), has led to intensive research into SARS-CoV-2, as well as its detection and treatment. Among the most important threads has been the need to detect new and emerging SARS-CoV-2 variants, since these are often associated with greater transmission and disease severity.
Amplicon-based sequencing methods have been used extensively in this regard. Yet, evidence of their utility is still lacking in completeness and strength.
 Study: Clinical Performance Characteristics of The Swift Normalase Amplicon Panel for Sensitive Recovery Of SARS-Cov-2 Genomes. Image Credit: Zita / Shutterstock.com
Study: Clinical Performance Characteristics of The Swift Normalase Amplicon Panel for Sensitive Recovery Of SARS-Cov-2 Genomes. Image Credit: Zita / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Millions of SARS-CoV-2 genomes are publicly available for analysis, using next-generation sequencing platforms that permit genomic surveillance under near real-life conditions. This includes whole-genome sequencing (WGS) of viral isolates from COVID-19-positive patients, which play a major role in investigating local outbreaks, planning interventions, developing preventive and therapeutic pharmaceutical agents, as well as assessing the effectiveness of such drugs.
SARS-CoV-2 variants of concern (VOCs) are new variants linked to higher transmissibility, reduced vaccine effectiveness, and adverse outcomes, usually because of specific sets of mutations that drive immune evasion and enhanced viral replication. Again, sequencing is key to identifying such mutations and developing monoclonal antibodies. Finally, the emergence of new strains in immunocompromised patients with chronic COVID-19 can be monitored by such technologies.
Multiplexed amplicon sequencing is preferable to shotgun sequencing or capture-based sequencing methods for its increased sensitivity, rapidity, and inexpensiveness. Earlier, the authors of the current study published on the medRxiv* preprint server demonstrated that such a panel could recover genomes up to a cycle threshold (Ct) over many isolates.
The Swift SNAP primer, which is the focus of the current study, is designed complementary to the ancestral Wuhan-Hu-1 genome and amplifies 345 amplicons over the entire ribonucleic acid (RNA) strand. This primer requires three hours or less to devise libraries of amplicons, from just 10 to 100+ copies of the virus. Further processing may be done manually or enzymatically using proprietary formulations.
Since guidelines for evaluating WGS assays are lacking for SARS-CoV-2, the current study used the guidelines of the United States Food and Drugs Administration (FDA) to validate the one-tube SARS-CoV-2 Swift Normalase Amplicon Panel (SNAP) Version 2.0 from Swift Biosciences.
This is the first time such validation is being carried out on this commercially available test that has been used in a number of vaccine trials and research studies.
Study findings
The researchers found that they were able to recover high-quality genomes from COVID-19 positive specimens with a 95% limit of detection of 40 SARS-CoV-2 copies per polymerase chain reaction (PCR), at concentrations of 82 copies/reaction or more. The genomes were recovered across a range of Ct values from about 11-37, with a median value of 22.
The researchers did not recover genomes from specimens that were negative by PCR or those which were positive for other viruses. They achieved pairwise identity for consensus sequences at 100% in all cases.
Replicated genomes recovered on the same run or on different runs were of good quality, in that allele frequencies were highly concordant between the Swift and shotgun sequencing libraries. The use of different instruments also yielded a high degree of reproducibility. The researchers found, in addition, that both VOC and non-VOC strains were properly identified, using benchmark datasets from the U.S. Centers for Disease Control and Prevention (CDC).
Mixtures of different clades of SARS-CoV-2 over a range of ratios led to the detection of the expected strains even at a ratio of 1:99. Once released for clinical use, the assay was used almost 270 times in the first 23 weeks. The median turnaround time was 11 days, and its use was mainly to investigate outbreaks and control infections.
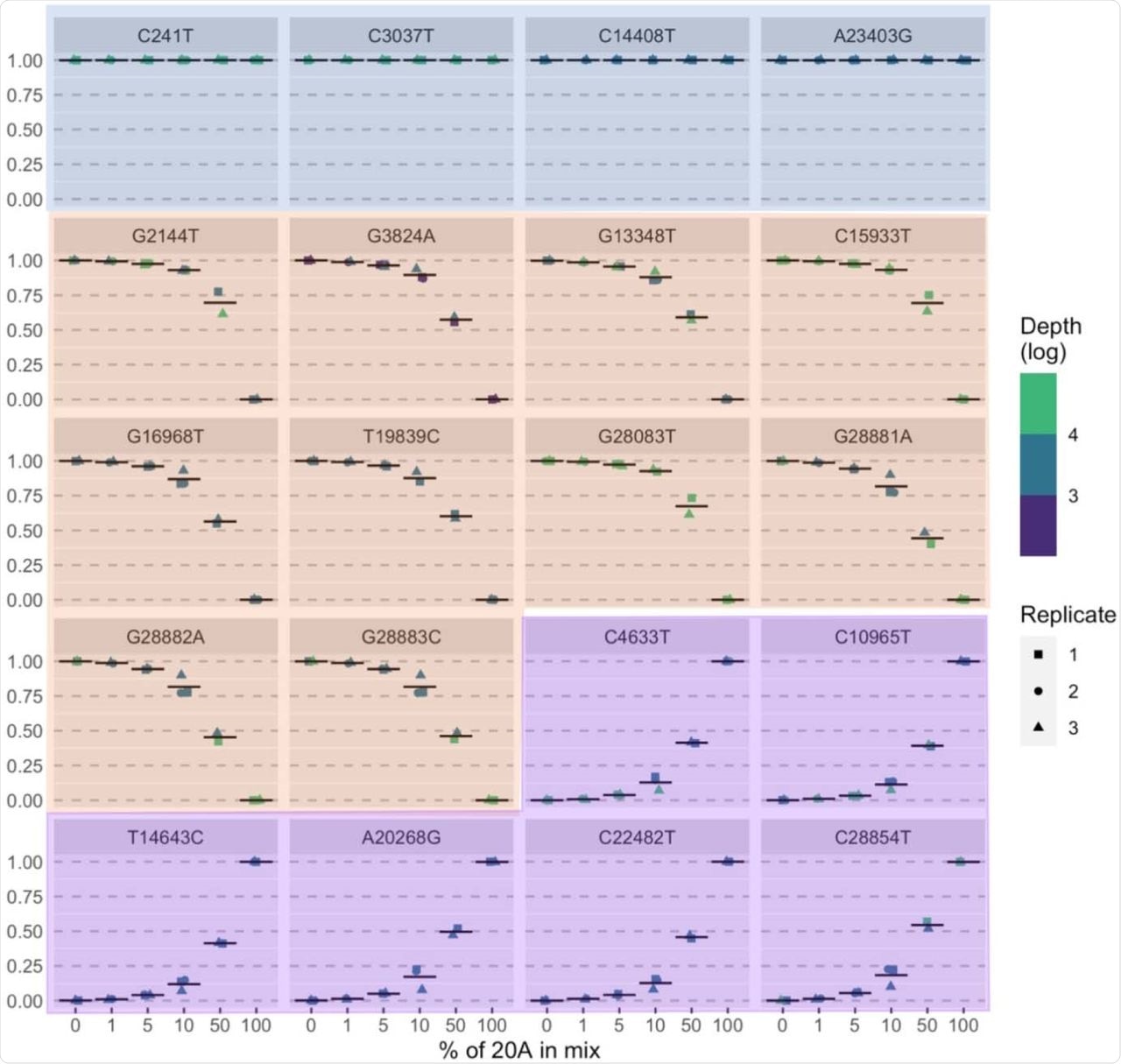 Measured allele frequency (y-axis) for all expected mutations (n =20) in sample mixtures described in Table 2. Blue panels = mutations common to 20A and 20B samples, orange panels = mutations expected in 20B sample, purple panel = mutations expected in 20A sample.
Measured allele frequency (y-axis) for all expected mutations (n =20) in sample mixtures described in Table 2. Blue panels = mutations common to 20A and 20B samples, orange panels = mutations expected in 20B sample, purple panel = mutations expected in 20A sample.
Implications
The findings of the current study confirm that multiplex PCR amplicon panels can be used to accomplish high-throughput sequencing of SARS-CoV-2. Not only can they be used to recover genomes over a large spectrum of viral loads, but they reduce workflow duration.
The validation of this platform using FDA guidelines that are applicable to other NGS tests in the current study shows that it can be used during routine clinical work in any Clinical Laboratory Improvement Amendments (CLIA) and College of American Pathologies (CAP)-approved laboratory. The approach discussed here was both highly sensitive, with a limit of detection of whole genomes at 40 copies per reaction, and found to accurately recover allele frequencies among mixed samples.
This is central to evaluating the variation of the SARS-CoV-2 lineage within a single host, as may occur after vaccination or treatment. In fact, amplicon sequencing may be more relevant in capturing alleles with minor frequencies compared to capture-based sequencing technologies.
The Swift SNAP panel can be directly used with short-read technologies as it produces amplicons of less than 255 base pairs (bp) as compared to others like ARTIC, which, though inexpensive, produce amplicons of greater than 400 bp. This means that further fragmentation is required before the use of Illumina-based sequencing or Oxford Nanopore Technologies (ONT)-based direct sequencing.
Since more primers are used in this panel, as well as overlapping amplicons, amplicon dropouts are relatively low. The ability of this panel under optimized manufacturer-recommended settings to recover genomes over a range of Ct values, as in real-life specimens, is another advantage.
The availability of an optional proprietary enzymatic normalization (Normalase) step can produce equimolar pools covering a range of samples and amplicons equally, thus making it simpler to handle samples in large batches rather than adding more amplification cycles. The latter may lead to low-quality runs because of excessive primer dimerization, as well as amplification of trace contaminants.
“Our results demonstrate the high sensitivity, specificity, and reproducibility of the Swift SNAP amplicon panel for SARS-CoV-2, which make it ideal for clinical applications.”
The researchers provide a link to the protocol at https://www.protocols.io/view/uw-virology-swift-snapv2-protocol-byw4pxgw. This can be used with robotic systems and is applicable for the validation of other amplicon sequencing methods.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Shrestha, L., Lin, M. J., Xie, H., et al. (2021). Clinical Performance Characteristics of The Swift Normalase Amplicon Panel for Sensitive Recovery Of SARS-Cov-2 Genomes. medRxiv. doi:10.1101/2021.10.22.21265255. https://www.medrxiv.org/content/10.1101/2021.10.22.21265255v1.
- Peer reviewed and published scientific report.
Shrestha, Lasata, Michelle J. Lin, Hong Xie, Margaret G. Mills, Shah A. Mohamed Bakhash, Vinod P. Gaur, Robert J. Livingston, et al. 2022. “Clinical Performance Characteristics of the Swift Normalase Amplicon Panel for Sensitive Recovery of Severe Acute Respiratory Syndrome Coronavirus 2 Genomes.” The Journal of Molecular Diagnostics : JMD 24 (9): 963–76. https://doi.org/10.1016/j.jmoldx.2022.05.007. https://www.jmdjournal.org/article/S1525-1578(22)00191-X/fulltext.