While the coronavirus disease 19 (COVID-19) pandemic is still ongoing, the global prophylactic vaccination drive has successfully achieved a remarkable milestone with approximately half of the world population having already received at least a first severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine dose.
 Study: Modelling the interplay of SARS-CoV-2 variants in the United Kingdom. Image Credit: Axel_Kock/Shutterstock
Study: Modelling the interplay of SARS-CoV-2 variants in the United Kingdom. Image Credit: Axel_Kock/Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
As the virus spreads, its genome replicates creating mutations that lead to virus variants. Several new variants have been identified in the SARS-CoV-2 genome, of which some clinically relevant variants (variants of concern), are transmitted more efficiently than the original ones and that can even cause more severe disease.
The most widely prevalent strains include the ones first detected in the United Kingdom (Alpha or B.1.1.7), South Africa (Beta or B.1.351), Brazil (Gamma or P.1), and India (Delta or B.1.617.2). The emergence of these new COVID-19 variants has resulted in the appearance of fast reinfections and co-infections and has raised doubts about the effectiveness of vaccines.
Given such global impact of the COVID-19 pandemic, a reliable model to analyze the dissemination of SARS-CoV-2 variants is crucial to explore effective mitigation strategies.
Effects of vaccination and non-pharmaceutical measures
In their recent work published on the medRxiv* preprint server, researchers from Argentina and Mexico have presented a geo-stochastic SEIRS-V model that provides a means of accurately describing the dynamics of the pandemic. The model, that reliably analyzes the spread of the different virus variants, has a unique property that it conveniently separates the parameters related to the disease from the ones related to social behavior and mobility restrictions, which enables to explain some global features observed in the daily number of worldwide cases. For instance, the model demonstrates that the surge of periodical waves is not only related to the appearance of new variants but is also associated with varying vaccination and social-distancing schemes.
The team highlighted that,
It consists of an extended compartmental model that includes various strains and vaccination strategies, allowing to study the emergence and dynamics of the new COVID-19 variants”.
The team uses this model to simulate the local dynamics of infection spreading, taking place in each cell of an area of a few km2, with average population density. Global dynamics, considers the geographical spread to neighboring cells and along terrestrial roads or aerial routes; governmental non-pharmaceutical interventions, and social behavior are reflected in the mobility parameters used.
Model application
In the current study, the team applied this model to the case of the United Kingdom (UK) to take advantage of the reliably available data. The information on density map and the main routes of country were extracted. The dynamics of different strains of the virus is very well documented in the UK, consequently the available information suffices to fit the parameters of the model for the different variants. Only the most abundant strains over time namely, EU1 strain (B. 1.177 originated in Europe), the Alpha (B.1.1.7) and the Delta (B.1.617.2) were considered in the adjustment. The rest of the less abundant variants were grouped together under the name "other strains".
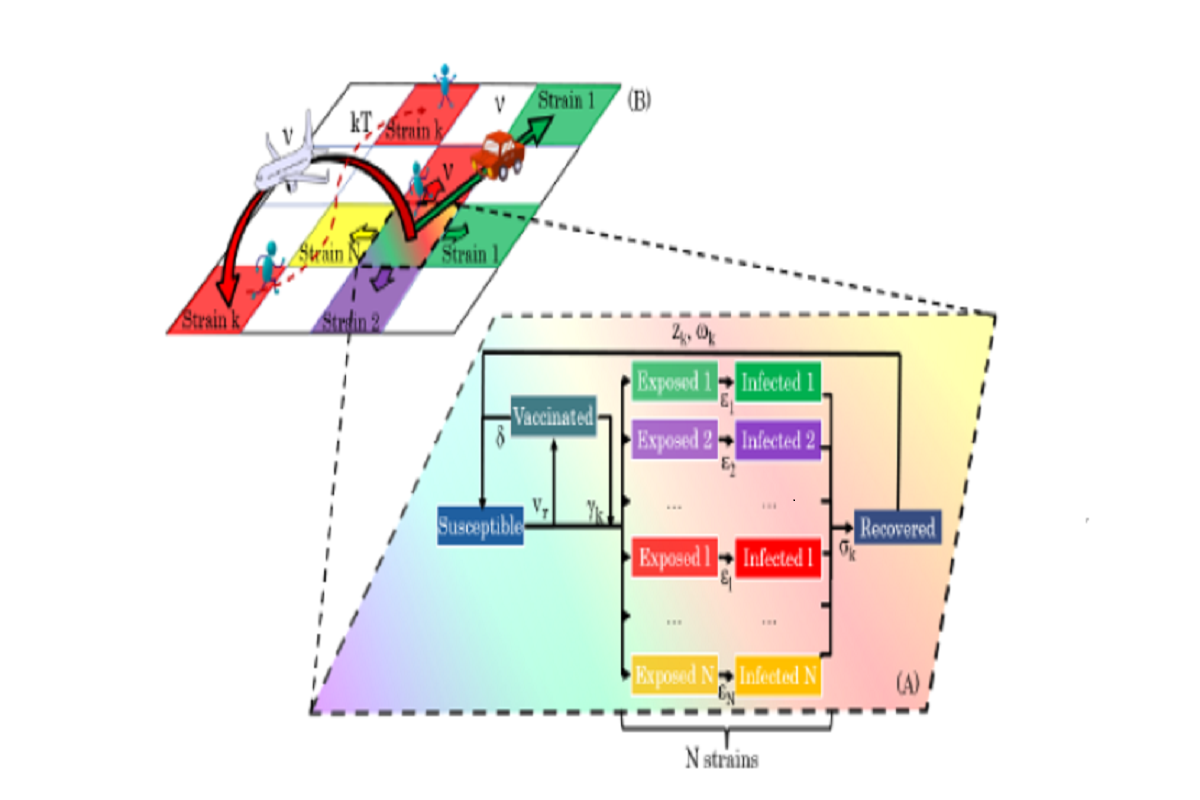
Figure 1: Diagram showing the geo-stochastic model scheme. (A) represents the local dynamics. Susceptible, Recovered, Vaccinated, Exposed and Infected with different strains, are taken into account. Vaccinated people can get infected with a certain variant k with a γk probability. The exposed, infectious and immune periods for the k− th strain are εk, σk and ωk, respectively. The variables vr and δ stand for the vaccination rate and the immunity period conferred by the vaccine. (B) The global dynamics on a geographical area, divided into a grid of cells, is followed by placing a SEIRS-V model on each one and allowing contagions between them. Three mobility processes are considered: movement to neighbor and far cells and thermal noise.
Restricting the spread of the virus
The model predicts for new COVID-19 waves in the future, if highly transmissible, vaccine-resistant variants are present. This would imply the need for new vaccines, including strain-specific proteins. However, mass vaccination is clearly essential to reduce the appearance of new variants of concern and, consequently, the need for those specific vaccines.
If vaccinated people get a mild version of the infection, COVID-19 will turn out to be a common, relatively harmless, influenza-like disease and there will be less susceptible people over time. Under that scenario, mobility restrictions should be applied mainly to contain the spread of new variants of concern and boosters should only be given to high-risk groups in the near future.
The results of the present study seem to imply that the world’s population would probably need to be vaccinated periodically in the years to come.
The team identified the epidemiological parameters which determine the dominant variant i.e. the one possessing a larger transmission coefficient or smaller exposed period before becoming infectious will eventually become dominant. Interestingly, as new variants with higher transmission coefficients appear, they quickly become the prevalent strain.
This model is useful to predict future scenarios, testing pharmaceutical and non-pharmaceutical interventions, and in particular to optimize the timing of vaccination boosters in order to minimize the appearance of new waves of the disease”, the team concludes.
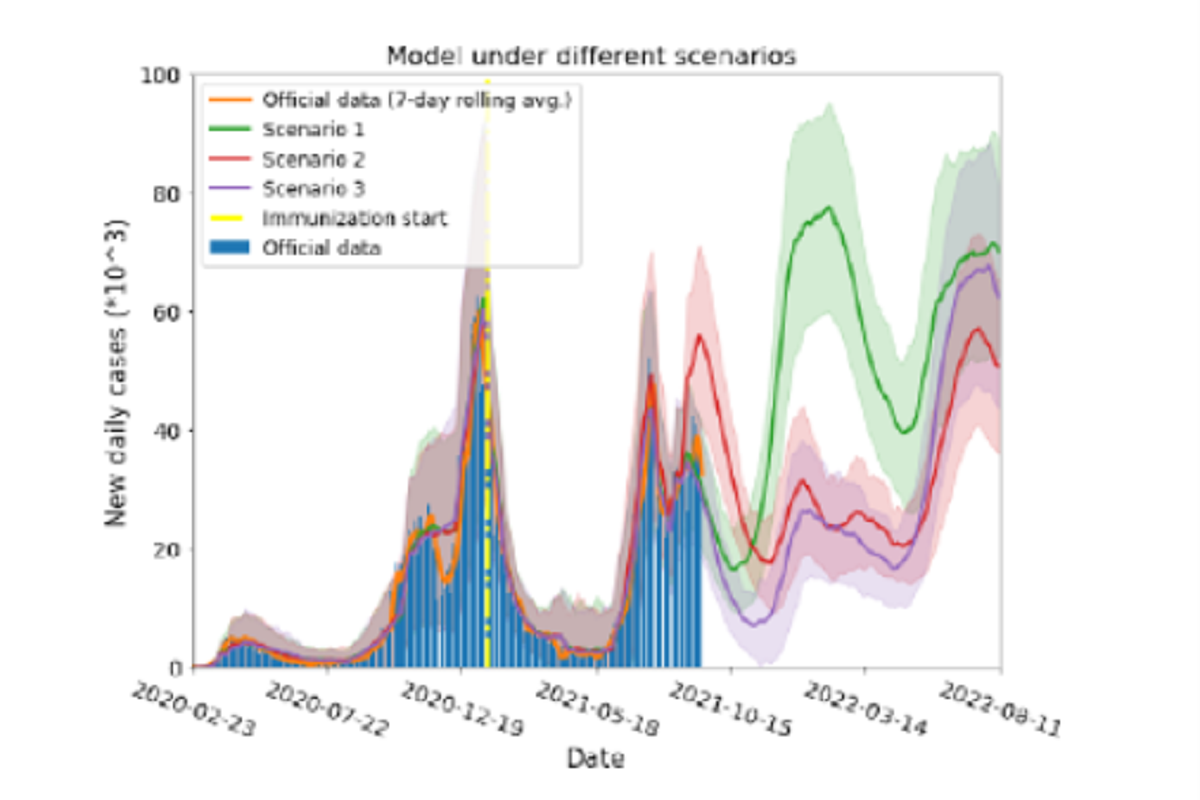
Figure 2: Model results for three different scenarios. Red, green and violet solid lines are the model simulation results under scenarios 1,2 and 3 (see text), respectively. Shaded areas on each color represent the standard deviation from 100 model runs for each scenario. Yellow dash-dot vertical line indicates the day when immunization started. Blue bars and solid orange lines are the actual daily cases and their 7 day rolling average, respectively. In scenario 1, a short vaccination immunity period implies a growth of the daily cases in the near future, if current mobility is sustained. Under scenario 2, people are expected to be immune for a longer time and breakthrough infections will act as antibodies boosters, prolonging the defence against the pandemic. In scenario 3 most people will be immune in the near future, lowering the number of cases, and a new wave would appear when vaccine immunity ends.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources