
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Further, the researchers also assessed NAb titers of individuals infected with SARS-CoV-2 before the emergence of the Omicron variant of concern (VOC) in individuals who were vaccinated with a single messenger ribonucleic acid (mRNA) vaccine dose. Although absolute NAb titer threshold is not an established correlate, this value is highly predictive of protection against SARS-CoV-2 infection and COVID-19.
Initially, COVID-19 mRNA vaccines were up to 95% effective against wild-type (WT) SARS-CoV-2 infection after the second dose, even among the elderly. However, follow-up data from vaccine effectiveness (VE) studies have shown waning of VE levels to 20% after five months of the second dose. For Omicron-induced COVID-19, VE estimates have shown rapid waning of immunity after two vaccine doses, which were restored after a booster dose.
About the study
The current observational clinical vaccine study included 20 HCWs and nine elderly patients in residential care homes. All study participants had received the first and second dose of the Comirnaty vaccine at a median of 21 days. The booster dose of either Comirnaty or Spikevax had been given at a median of 245 days following the second dose. Following the booster dose, blood samples were collected at a median of 40 days.
The researchers measured NAb titers against a WT SARS-CoV-2 strain, as well as the Beta (B.1.351), Delta (B.1.617.2), and Omicron (B.1.1.529) variants in HCWs and elderly subjects who received a booster vaccination six to nine months after the second dose.
NAb responses against the Alpha, Beta, Delta and Omicron variants to a single vaccination were also measured in 38 subjects infected before the Omicron variant was detected. These responses were compared to their NAb titers to the peaking antibody response one month after reinfection.
Study findings
The anti-spike (S) immunoglobulin G (IgG) geometric mean concentrations (GMCs) 21 to 42 days following a Comirnaty booster dose increased by 1.4-fold against the receptor-binding domain (RBD) and 1.8-fold against the full-length spike glycoprotein (SFL) in HCWs as compared to the elderly. The IgG GMCs remained equally high in HCWs at 43 to 77 days after the booster, including against the Omicron variant.
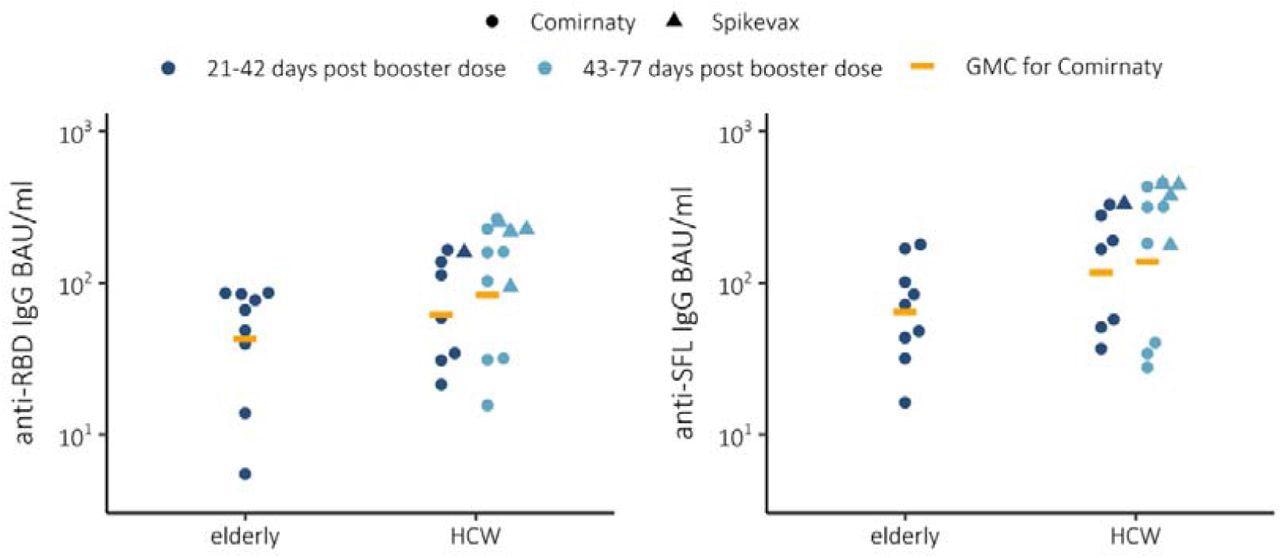
IgG concentrations and geometric mean concentrations (GMC) expressed as BAU/ml for spike proteins (SFL and RBD) in elderly (n=9) and health care workers (HCW) 21-42 (n=8) or 43-77 (n=12) days post booster mRNA vaccination (3rd dose) using Comirnaty. Booster mRNA vaccination Spikevax vaccinated HCWs 21-42 (n=1) and 43-77 (n=4).
However, among the elderly, the antibody concentrations measured a month after the booster dose was low. Furthermore, not all of the elderly study participants had NAbs against the Beta and Omicron variants.
Vaccination-induced antibody responses were highest in younger HCWs and lowest in individuals over 65 years. Moreover, NAb concentrations declined faster with increasing age, thus suggesting that protection against breakthrough infections following a booster dose may be incomplete and short-lived in the elderly.
The study findings also suggest that booster vaccination is most likely dose-dependent. Hence the currently licensed Spikevax booster dose of 50 μg showed a marked increase in NAb titers against Omicron in HCWs previously immunized with two doses of the Spikevax vaccine. Furthermore, the HCWs who received a full dose of a Spikevax booster had 2.2-fold (RBD) to 2.5-fold (SFL) greater IgG GMCs as compared to those vaccinated with Comirnaty.
In HCWs vaccinated with two doses of an mRNA vaccine, Spikevax induced two-fold greater antibody concentrations compared to Comirnaty. However, 20% and 24% of the subjects vaccinated with Comirnaty had NAbs against the two Omicron variants tested as compared to 100% against the Beta and the Delta variants.
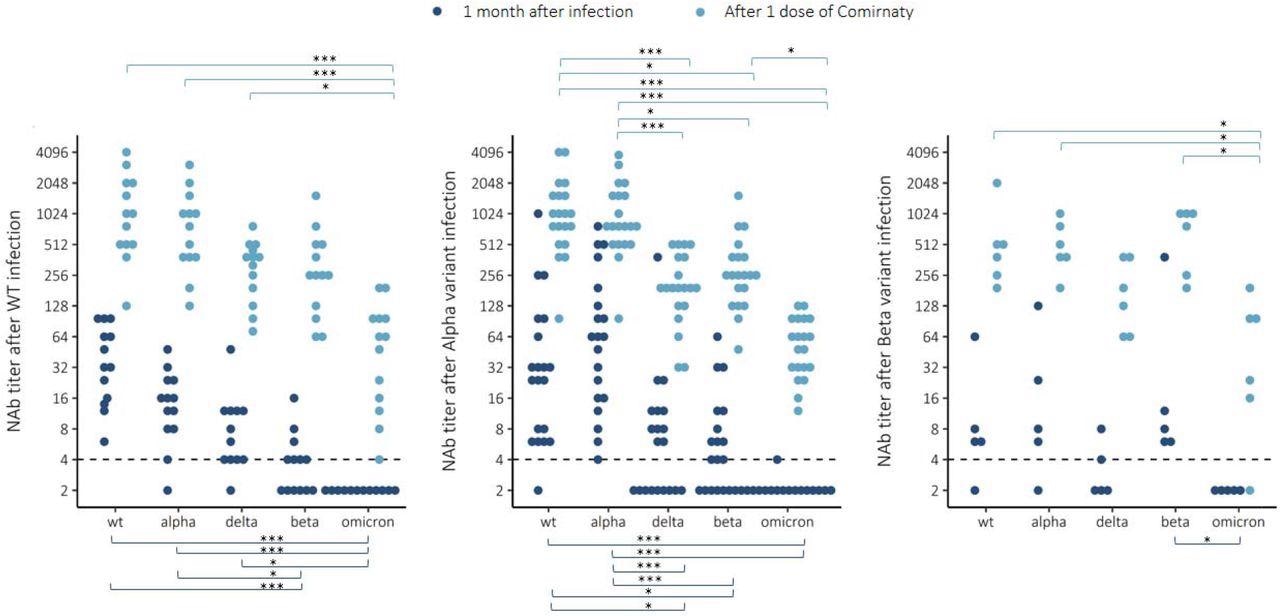
Neutralizing antibody (NAb) titers to wild-type (WT) virus and 4 variants Alpha (B.1.1.7), Delta (B.1.617.2), Beta (B.1.351) and Omicron (B.1.1.529) 1 month after infection with WT, Alpha or Beta variant and after one dose of Comirnaty COVID-19 vaccine. Wilcoxon rank-sum test *=P<0.05, **=P<0.01, ***=P<0.001.
The kinetics of antibody responses and the extent of cross-protective immunity following infection and vaccination may vary qualitatively. To this end, it was observed that anti-S IgG concentrations among previously infected and once-vaccinated HCWs were high and NAb titers variable as compared to the antibody concentrations following a third vaccine dose.
In vaccinated HCWs, a booster dose induced a memory B-cell response. In addition, in previously infected HCWs, a single mRNA vaccine dose induced higher NAb concentrations and higher antibody affinity by recalling pre-existing memory B-cells that showed rapid affinity maturation, which correlated with improved NAb titers against multiple SARS-CoV-2 variants.
However, as the level of NAbs declined over time, especially against antigenically different strains such as the Omicron variant, the third booster dose increased the concentration of antibodies. In addition, it improved the quality of the B cell response. As a result, this additional dose increased the potential long-term immunity against severe COVID-19.
Conclusions
The current study findings support previous findings that suggest booster vaccinations raise IgG concentrations against SARS-CoV-2. However, NAb titers remained low against the Omicron variant as compared to the WT and Delta strains of SARS-CoV-2.
The current study also demonstrated measurable NAbs against the SARS-CoV-2 Delta, Beta, and Omicron variants up to 2.5 months after the third vaccine dose in younger subjects. In contrast, some elderly subjects did not have antibodies against the Beta and Omicron variants, even one month after the third dose.
Insignificant and barely detectable NAb titers against Omicron one month post mild SARS-CoV-2 infection caused by WT, Alpha, or Beta strains suggested an increased risk of Omicron-induced reinfection. However, a single mRNA COVID-19 vaccine dose in previously infected individuals induced NAb titers comparable to the third dose of mRNA COVID-19 vaccination.
Taken together, the study findings indicate that booster vaccinations improved protection at least temporarily, which might have helped in reducing SARS-CoV-2 transmission during the pandemic. Furthermore, booster vaccination-induced long-lasting memory B-cells and T-cell-mediated immunity against severe COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Haveri, A., Solastie, A., Ekstrom, N., et al. (2021). Neutralizing antibodies to SARS-CoV-2 Omicron variant after 3rd mRNA vaccination in health care workers and elderly subjects and response to a single dose in previously infected adults. medRxiv. doi:10.1101/2021.12.22.21268273. https://www.medrxiv.org/content/10.1101/2021.12.22.21268273v1.
- Peer reviewed and published scientific report.
Haveri, Anu, Anna Solastie, Nina Ekström, Pamela Österlund, Hanna Nohynek, Tuomo Nieminen, Arto A. Palmu, and Merit Melin. 2022. “Neutralizing Antibodies to SARS-CoV-2 Omicron Variant after Third MRNA Vaccination in Health Care Workers and Elderly Subjects.” European Journal of Immunology, March. https://doi.org/10.1002/eji.202149785. https://onlinelibrary.wiley.com/doi/10.1002/eji.202149785.