Background
The recent outbreak of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused unprecedented damages to the global healthcare system. Several control measures, including facemask wearing, social distancing, movement restrictions, and lockdowns, were implemented locally and nationally to control the viral transmission. Several nanoparticle vaccines have been produced in record time and speed to reduce COVID-19 related morbidity and mortality.
The application of nanotechnology has revolutionized modern medicine in many ways, including drug design and delivery as well as the development of diagnostics and therapeutics. Most importantly, Pfizer–BioNTech’s and Moderna’s nanotechnology-enabled mRNA vaccines are the first of their kind to be approved for human use for COVID-19.
Nanoparticle-based approaches are vital for targeted delivery of genetic materials or antigenic proteins to antigen-presenting cells, leading to controlled immunogenic responses. The most commonly used nanoparticles in nano-vaccination systems include lipid, inorganic, polymeric, and virus-like nanoparticles.
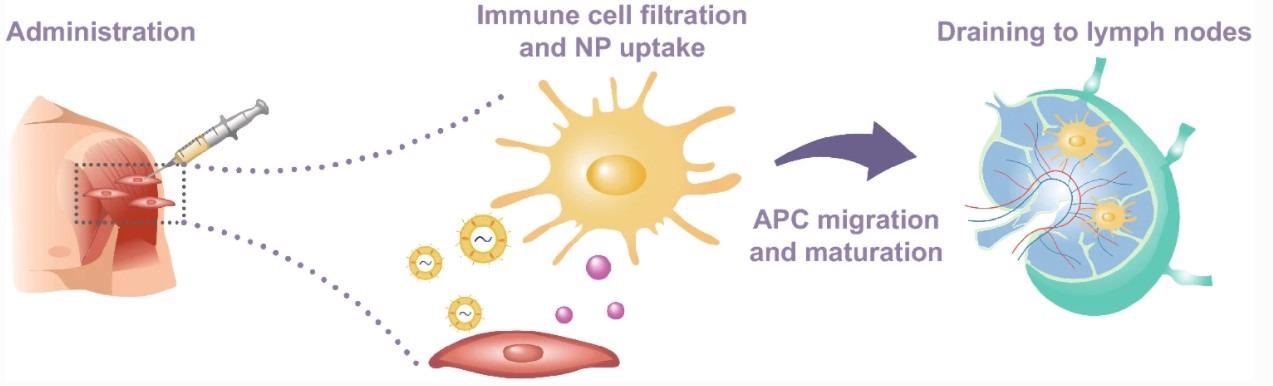
Schematic representation of vaccine administration, nanoparticle uptake by immature APCs, and subsequent migration to lymph nodes through the lymphatic system
Lipid-based nanoparticles are most suitable for encapsulating therapeutics, including proteins, nucleic acids, and small molecules. The main advantages include easy formulation and synthesis, biocompatibility, and high bioavailability.
Fabrication of polymeric nanoparticles can be performed with both natural (chitosan, pectin, dextran) and synthetic (polyacrylates, polylactic acid, poly lactic-co-glycolic acid) materials. Polymeric nanoparticles are suitable for delivering drugs, proteins, or genetic materials conjugated to the polymer and immobilized into its matrix or attached to the polymer surface.
Inorganic nanoparticles (gold, iron oxide, etc.) are suitable for immunological applications because of their unique electrical, optical, and magnetic properties. The biocompatibility of antibodies and other therapeutics can be increased by attaching them to inorganic nanoparticles through surface modification.
Virus-like nanoparticles mimic the structural conformation of wild-type viruses. Purified viral proteins are used to synthesize these nanoparticles. The application of virus-like nanoparticles in vaccination platforms has several advantages, including increased uptake of vaccine materials and induction of specific immune responses.
Nanoparticle-based COVID-19 vaccines
So far, two nanoparticle-formulated, mRNA-based COVID19 vaccines have been developed, clinically tested, and marketed during the ongoing pandemic. These vaccines are BNT162b2 by Pfizer/BioNTech and mRNA-1273 by Moderna.
In nucleic acid-based vaccines, viral genetic information is delivered to host cells in the form of plasmid DNA or mRNA (messenger RNA), which encode viral antigens and induce desired host immune responses. Nanoparticle-based encapsulation of genetic material is an attractive strategy to improve vaccine delivery as it protects enzymatic degradation of genetic material and facilitates the crossing of materials through cellular and nuclear membranes.
Nucleic acid-based nanovaccines are developed by mixing plasmid DNA or mRNA with lipids or polymers to create electrostatic complexes. The complex is further encapsulated into a nanoparticle system.
During cellular delivery of vaccine materials, nanoparticle shell fuses with the cellular membrane, leading to the release of genetic materials directly into the cytoplasm. After delivery, genetic materials undergo ribosomal translation to generate antigenic proteins, which are subsequently presented on the surface of antigen-presenting cells and recognized by circulating T cells. Upon activation, T cells secrete cytokines and activate B cells to produce antigen-specific antibodies.
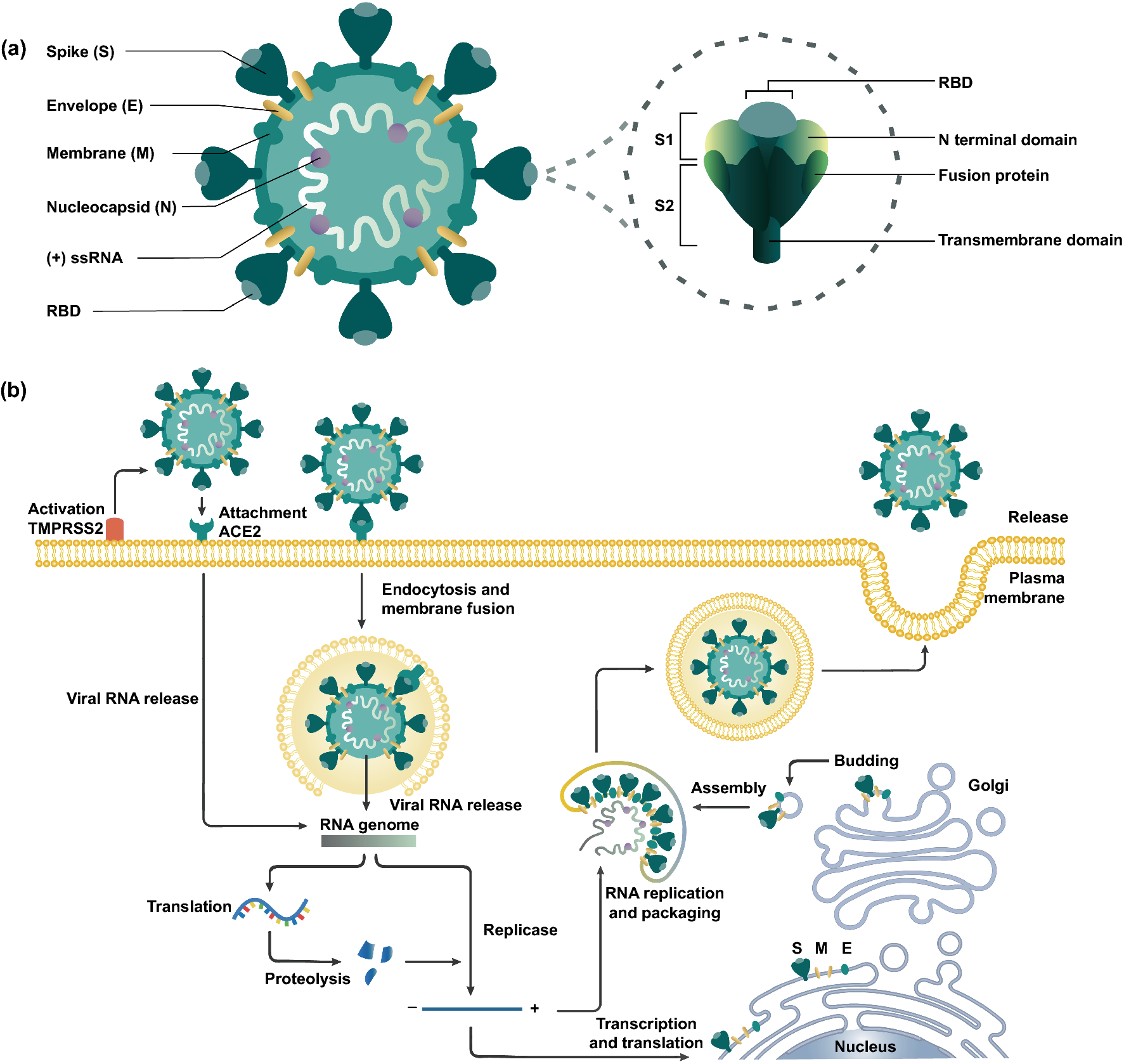
a Schematic representation of SARS-CoV-2 and spike glycoprotein main structural features. b The viral replication cycle initiates by the activation of the serine protease TMPRSS2 and angiotensin-converting enzyme 2 (ACE2) receptors
mRNA-based COVID-19 vaccine by Pfizer/BioNTech
The COVID-19 vaccine BNT162b2 developed by Pfizer/BioNTech is a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine that contains prefusion-stabilized, membrane-anchored full-length spike protein of SARS-CoV-2 as a vaccine antigen.
In phase II/III human clinical trials, two doses (30 µg each) of the vaccine were administered at a fixed interval of 21 days to more than 40,000 adults from 152 locations across the globe. The trial results showed that the vaccine to be 95% effective in preventing SAR-CoV-2 infection and 52% effective in preventing severe disease. According to the US Food and Drug Administration (FDA) criteria, a vaccine can be authorized for use if it has a primary efficacy of more than 30%.
After vaccination, the most commonly detected reactions were injection site pain, fatigue, and headache. In addition, severe local and systemic reactions were observed in 0.6% of vaccine recipients, which resolved within a couple of days.
In the current real-world pandemic situations, a reduction in vaccine efficacy has been observed mainly because of the emergence of new viral variants. Therefore, to improve vaccine efficacy, many countries across the globe have considered a third booster dose.
mRNA-based COVID-19 vaccine by Moderna
The mRNA-1273 COVID-19 vaccine developed by Moderna is a lipid nanoparticle-encapsulated mRNA-based vaccine that encodes prefusion-stabilized full-length SARS-CoV-2 spike with two proline substitutions at the residues 986 and 987.
In the phase III clinical trial, two doses (100 µg each) of the vaccine were administered at a fixed interval of 28 days to more than 30,000 adults. The trial results showed that the vaccine is 94% effective in preventing SARS-CoV-2 infection. The most commonly observed reactions after vaccination were injection site pain, fatigue, and headache. Severe reactions were observed in some recipients, which resolved within a couple of days.
In both Pfizer and Moderna vaccines, lipid nanoparticles are used to encapsulate mRNA. These nanoparticles are biodegradable and can act as adjuvants to boost vaccine efficacy. To overcome the disadvantages of mRNA instability and immunogenicity, chemical modifications of nucleotides have been undertaken in both vaccines.
In order to combat this pandemic, a rapid and multidisciplinary response was necessary. Biomedical nanotechnology, or nanomedicine, as it is more commonly known, is at the intersection of engineering and biology and offers researchers the ability to develop novel nanoscopic strategies for combating ever-evolving pathogens. For example, many biotechnology companies and academic institutions have chosen nanotechnology to design and develop mRNA vaccines against SARS-CoV-2. At present, over 26 nanoparticle vaccine candidates have advanced into clinical testing, with ∼60 more in pre-clinical development. It is now evident that nanotechnology has evolved into a pivotal field in terms of preventing further deaths, curtailing the pandemic, and assisting in the economic recovery of the world.