As of February 22, 2022, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected over 427 million individuals and caused over 5.7 million deaths. Despite high vaccination coverage, antigenic drift variants are an unavoidable concern and therefore pose significant threats to global health.
Study: Mosaic receptor-binding domain nanoparticles induce protective immunity against SARS-CoV-2 challenges. Image Credit: Dotted Yeti / Shutterstock.com

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Construction of mosaic nanoparticles
As a scaffold, a heterotrimeric PCNA from S. solfataricus with three different subunits of PCNA1, 2, and 3 were used for the current study. Despite their modest sequence similarity that was within the range of 8-22%, these 250 amino acid subunits have similar structures and are assembled sequentially. To this end, PCNA1 and PCNA2 form a stable dimer, which utilizes PCNA3 to form a heterotrimer that has dissociation constants within the micromolar (µM) to millimolar (mM) range.
The construction of the three plasmids S-PCNA1, M-PCNA2, and H-PCNA3 were based on the fusion protein sequences obtained from the SARS-CoV-2, Middle East Respiratory Syndrome CoV (MERS-CoV), and human CoV HKU1 (HKU1), respectively. S-PCNA1, M-PCNA2, and H-PCNA3 were isolated using His-tag affinity, ion exchange, and size exclusion chromatography after being expressed in HEK 293F cells.
S-PCNA1, M-PCNA2, and H-PCNA3 were mixed sequentially to form mosaic multivalent nanoparticles that were referred to as 6RBD-np. The assembly, size, and morphology of 6RBD-np were further characterized through the use of atomic force microscopy (AFM) and transmission electron microscopy (TEM).
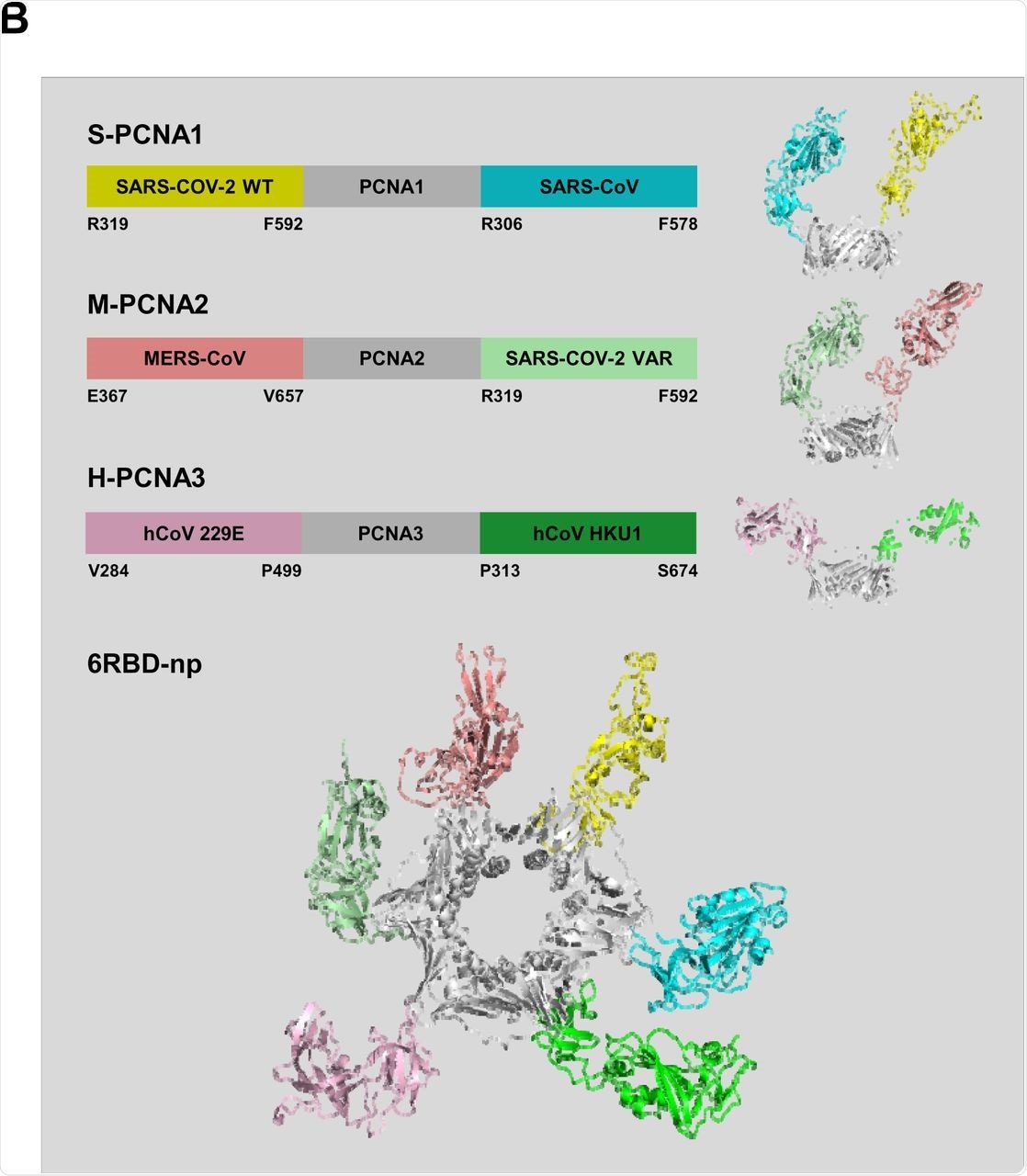
Design and characterization of 6RBD-np antigen. (A) Schematic of SARS-CoV-2 spike primary structure colored by domains (upper panel). S1 region contains NTD, N-terminal domain; RBD, receptor binding domain; SD1 and SD2, subdomain 1 and 2, and S2 region. The mutation sites for SARS-CoV-2 variants are indicated by yellow vertical lines. The RBD and SD1 regions are derived from from 319 to 592 for SARS-CoV-2 WT and variant, 306 to 578 for SARS-CoV, 367 to 657 for MERS-CoV, 313 to 674 for hCoV-HKU1, and 284 to 499 for hCoV-229E spike proteins. A prefusion trimeric structure of SARS-CoV-2 spike protein (lower panel). A single protomer is shown in ribbon representation and colored as in the primary structure diagram. The two remaining protomers are shown as molecular surface in gray. (B) Design of S-PCNA1, M-PCNA2 and H-PCNA3 showing schematic diagrams of CoV spike RBD-PCNA fusion proteins (upper panel): S-PCNA1, M-PCNA2 and H-PCNA3. S-PCNA1, SARS-CoV-2 RBD-SD1 WT-PCNA1-SARS-CoV RBD-SD1; M-PCNA2, MERS-CoV RBD-SD1-PCNA2-SARS-CoV-2 RBD-SD1 VAR; H-PCNA3, hCoV 229E RBD-SD1-PCNA3-hCoV HKU1 RBD-SD1. Composite molecular models of S-PCNA1, M-PCNA2 and H-PCNA3 are shown on the right. A molecular model of 6RBD-np displaying PCNA ring (gray) and 6 RBD-SD1 proteins colored as in the schematic diagram (lower panel). The models were generated from structures of PDB IDs: 6VXX, 5W9P, 5X5B, 6U7H, 5I08, and 2HIK for SARS-CoV-2, MERS-CoV, SARS-CoV, hCoV 229E, and hCoV HKU1 spike proteins, and PCNA, respectively. (C) Purified 6RBD-np was characterized using SEC-MALS to show its molecular weight of 355.7 kDa, suggesting a stable self-assembled 6RBD-np.
Efficacy of 6RBD-np in vivo
The immune response generated by 6RBDnp was evaluated in BALB/c mice that received two intramuscular injections of 6RBD-np at an interval of three weeks. Blood samples were collected two weeks after the prime immunization, as well as two and four weeks after the second booster dose.
Mice that received S-PCNA1 alone, as well as those that received 6RBD-np exhibited the highest antibody titers after their booster dose, thereby demonstrating that a robust antibody response was achieved by these two treatments.
Further investigation following challenge with SARS-CoV-2, SARS-CoV, and hCoV HKU1 demonstrated that both S-PCNA1 and 6RBD-np treated mice produced high antibody titers against SARS-CoV-2 and SARS-CoV, whereas 6RBD-np was the only treatment that successfully induced antibody titers against HKU1. The 6RBD-np treatment was also found to neutralize the entry of pseudoviruses expressing the SARS-CoV spike protein, as well as the SARS-CoV-2 spike proteins containing the D614G, Delta, or SD1 mutations.
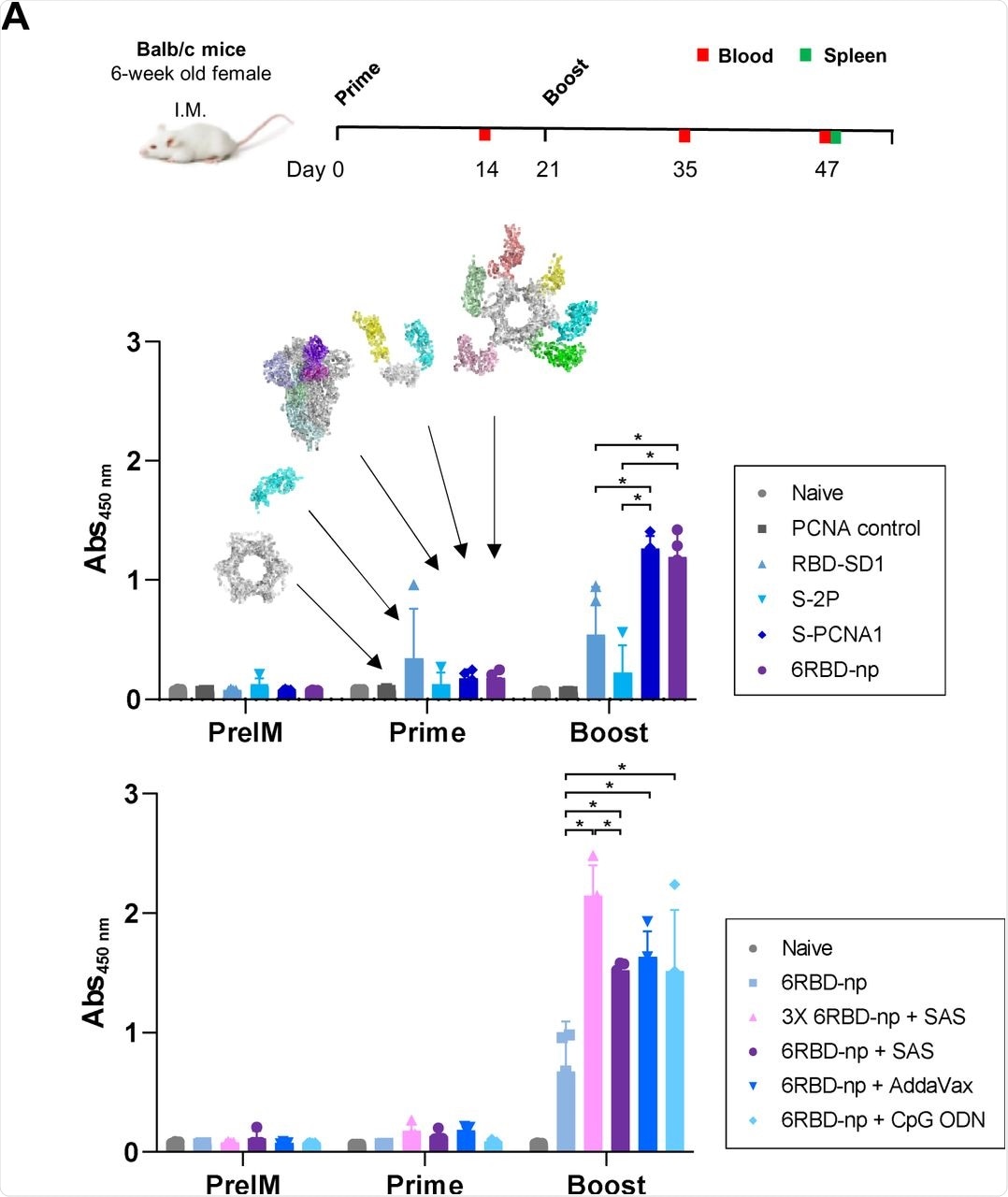
(A) Prime-boost immunization study design with BALB/c mice (upper panel). The mice were immunized in six groups. Blood was collected two weeks after the prime and boost immunizations and 26 days after the boost. Post-prime and post-boost anti-RBD Ab titers in BALB/c mice, for different immunogens of the groups of the naïve and PCNA controls, RBD-SD1 of SARS-CoV-2, S-2P of SARS-CoV-2, S-PCNA1, and 6RBD-np, represented as molecular structures of the respective immunogens with arrows (middle panel). Additional experiments were performed for different doses and adjuvants (lower panel), in groups of 5 μg and 15 μg of 6RBD-np, 5 μg of 6RBD-np in the presence of SAS, AddaVax, and CpG-ODN. The 15 μg 6RBD-np group shows the highest Ab response, compared to other groups.
Mice that were genetically engineered to express the human angiotensin-converting enzyme 2 (hACE2) receptor, which is required for cellular entry of SARS-CoV-2, were then treated with two immunizations at three weeks apart. Forty-two days after their first dose, all mice were challenged with an intranasal inoculation of SARS-CoV-2 wild-type (WT) or Delta.
Mice that received treatment with S-2P, S-PCNA1, and 6RBD-np and were subsequently challenged with the SARS-CoV-2 WT exhibited 100% survival rates. Comparatively, mice that received the RBD-SD1 and 6RBD-np treatment prior to Delta exposure exhibited 100% survival as well.
Notably, 6RBD-np treatment produced 100% survival rates following exposure to both SARS-CoV-2 WT and Delta strains. The lungs and brains of mice treated with 6RBD-np also exhibited lower viral titers at 5 days post-infection (dpi) with both the WT and Delta variant challenges.
Both S-PCNA1 and 6RBD-np treatments also produced high neutralizing antibody responses in the serum after exposure to both SARS-CoV-2 WT and Delta strains.
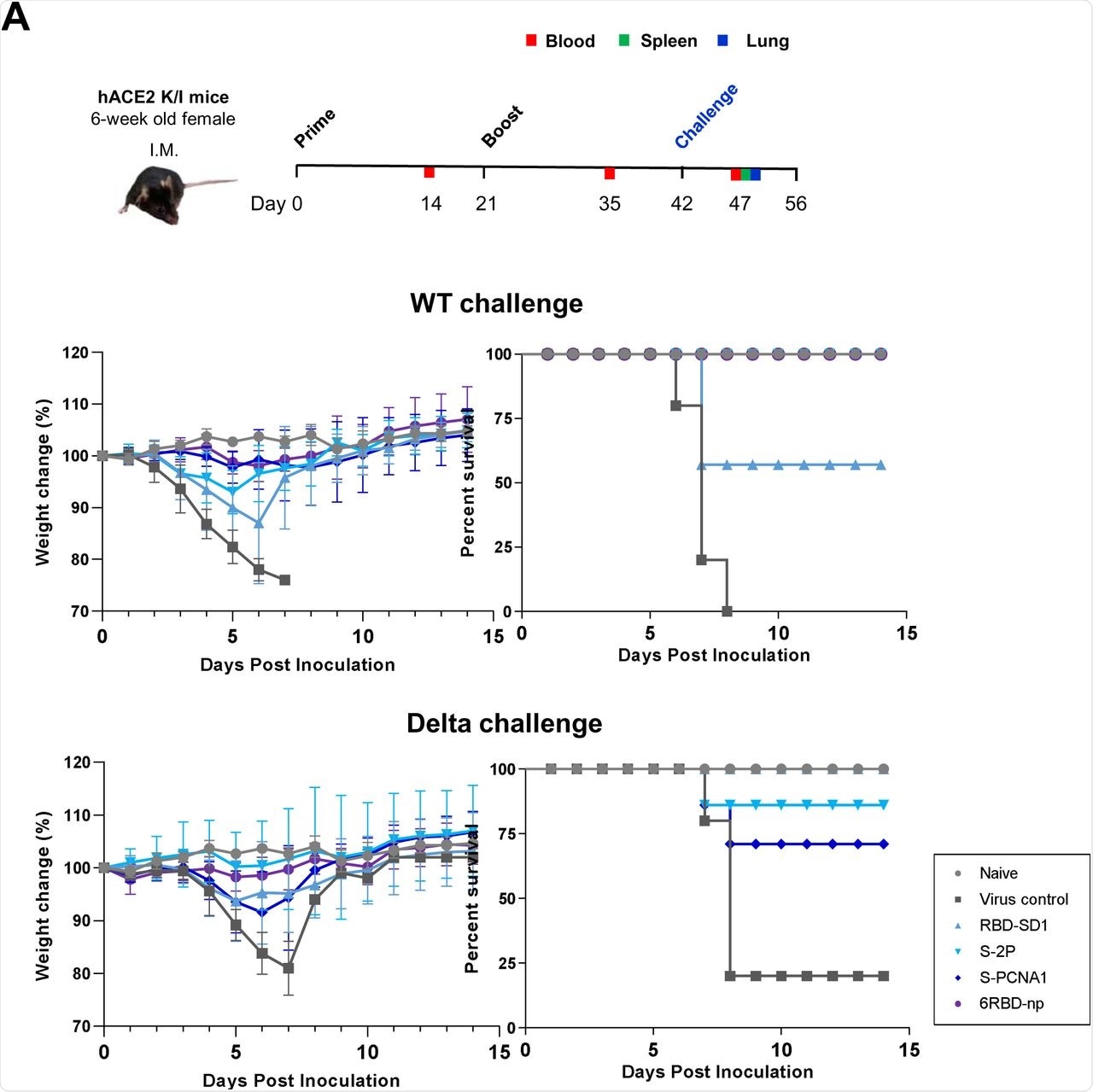
Protective efficacy and immunogenicity of antigens against SARS-CoV-2 WT and Delta challenges in hACE2 transgenic mice. (A) Prime-boost immunization and challenge study design with hACE2 transgenic mice (upper panel). Weight loss and survival of hACE2 transgenic mice, following the SARS-CoV-2 WT or Delta challenge up to 14 days post-infection, in groups of RBD-SD1 and S-2P of SARS-CoV-2, S-PCNA1, and 6RBD-np (n=10), including the naïve (n=6) and virus-infected control (n=8) groups. The mosaic 6RBD-np group shown in violet demonstrates little changes in body weight and survival, the only group showing survival rates of 100%, regardless of the virus strains (middle and lower panels). The naïve mice are shown by a blue line.
Future perspectives
Taken together, the findings from the current study demonstrate that 6RBD-np treatment produced significant and dose-dependent antibody responses and induced complete protection against both the SARS-CoV-2 WT and Delta challenges in BALB/c and hACE2-transgenic mice. These findings demonstrate the potential utility of this mosaic nanoparticle to co-display heterologous antigens that could be used for innovative vaccine designs in the future.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.