Several variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have emerged since the virus was originally identified in late December 2019.
Background
More recently, the SARS-CoV-2 Omicron variant BA.1 (B.1.1.529) emerged in late 2021 and was quickly designated as a variant of concern (VOC) in November 2021 by the World Health Organization (WHO) as a result of its numerous mutations in its spike (S) protein and notable vaccine escape properties. Epidemiological studies have demonstrated that two doses of coronavirus disease 2019 (COVID-19) vaccines, along with a booster dose of a messenger ribonucleic acid (mRNA) vaccine, confers temporary protection against Omicron BA.1.
Several countries where BA.1 was initially detected reported that the Omicron BA.1 lineage was promptly replaced by the Omicron BA.2 sub-lineage. To date, the Omicron BA.2 variant is the current dominant circulating strain of SARS-CoV-2, as it represents nearly 86% of all newly sequences cases.
The United States, for example, has recently reported a weekly doubling of cases with BA.2 sub-lineage. The increased infection rates and transmissibility in countries with high vaccination rates indicate that the BA.2 sub-lineage harbors a more potent vaccine escape mechanism as compared to the Omicron BA.1 lineage.
About the study
A recent CDC Emerging Infectious Diseases study investigates the Omicron BA.2 sub-lineage for its vaccine escape capabilities by comparing the neutralization of BA.1 and BA.2 strains in samples from immunized persons who had received two doses and a booster dose of the Pfizer-BioNTech BNT162b2 mRNA vaccine. All samples were collected between January 26, 2022, to January 28, 2022.
Serum samples were analyzed with a 90% plaque reduction neutralization test (PRNT90 ). Clinical isolates for PRNT90 were identified by whole-genome sequencing, which provided information on both the lineage and mutation calls in the samples. The presence of neutralizing antibodies was also analyzed in the samples using the Liaison TrimericS immunoglobulin g (IgG) Quantitative immunoassay.
Study findings
The study cohort consists of 20 immunocompetent participants, which included 12 females and eight males, without any prior history of a prior SARS-CoV-2 infection. The median age of the participants was 57 years.
The median time between the two mRNA vaccine doses was 35 days, whereas the median time between the second dose and booster dose was 168 days. The median time between the booster dose and sample collection was 42 days.
The neutralization titers within the serum samples exceeded the threshold limit for both the Omicron BA.1 and the BA.2 sub-lineage. Statistically significant differences were reported between the neutralization titers of BA.1 and BA.2 as compared to the ancestral strain; however, the difference in neutralization titers was insignificant between BA.1 and BA.2.
Vaccine evasion appeared to be the primary mechanism contributing to the higher transmissibility of the Omicron BA.1 and BA.2 sub-lineage. Nevertheless, BA.2 was not able to evade the humoral immunity induced by the BNT162b2 vaccine any better than BA.1.
It was thereby inferred that the Omicron BA.2 sub-lineage has divergent modes of transmissibility other than vaccine-induced antibody escape that have contributed to the current upsurge of BA.2 infections.
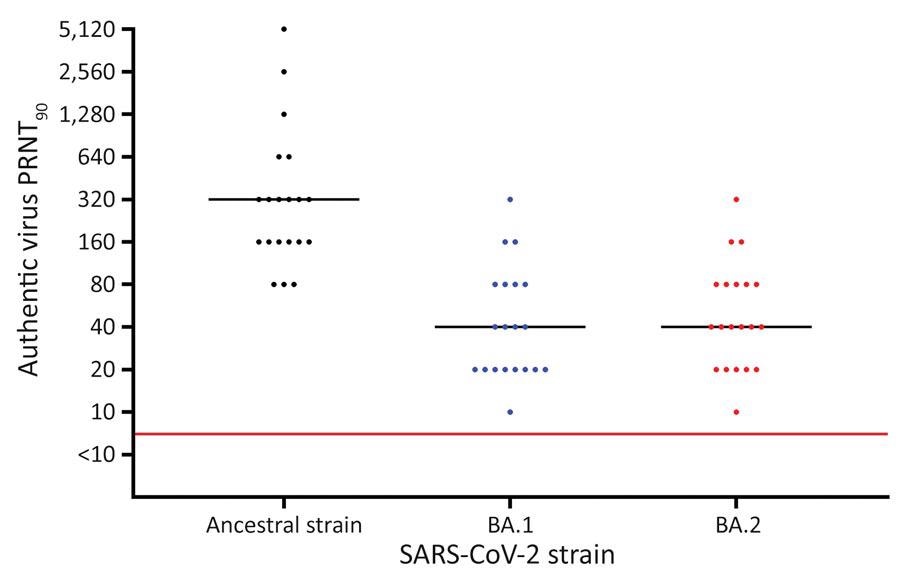
Results of PRNT90 of serum against SARS-CoV-2 ancestral strain and Omicron sublineages BA.1 and BA.2 after BNT162b2 (Pfizer-BioNTech, https://www.pfizer.com) booster vaccination, Denmark. Serum samples were collected from 20 SARS-CoV-2–naive participants who received 2 BNT162b2 doses and a booster BNT162b2 dose. Viral genome sequences are available in GenBank (accession nos. ON055855 for the ancestral strain, ON055874 for BA.1, and ON055857 for BA.2). Red line indicates neutralization threshold; black lines indicate median neutralization titers for each strain. PRNT90, 90% plaque reduction neutralization test.
Conclusions
The neutralization of the Omicron BA.1 lineage and BA.2 sub-lineage are equal, as their neutralization capabilities appear to be nearly eight times lower when compared to the ancestral strain of SARS-CoV-2. The threshold neutralization value for BA.1 and BA.2 six weeks after a booster vaccination indicated at least transient protection conferred by the vaccine against mild infection by the BA.2 sub-lineage.
Taken together, the findings from the current study emphasize the importance of booster vaccination after two prior COVID-19 vaccine doses, as it confers protection against both Omicron BA.1 and BA.2 strains.
Journal reference:
- Pedersen, R. M., Bang, L. L., Madsen, L. W., et al. (2022). Serum neutralization of SARS-CoV-2 Omicron BA.1 and BA.2 after BNT162b2 booster vaccination. The United States Centers for Disease Control and Prevention Emerging Infectious Diseases. doi:10.3201/eid2806.220503.