Coronavirus disease 2019 (COVID-19) cases have significantly increased dramatically following the emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron (B.1.1.529) variant. Although booster messenger ribonucleic acid (mRNA) vaccine doses offer protection against severe COVID-19 induced by the Omicron variant, the ability of this third vaccine dose to prevent infection and viral transmission appears to be limited and insufficient.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The probability of infection with the wild-type strain and other SARS-CoV-2 variants of concern (VOCs) was significantly linked with vaccine-induced serological responses. However, little is known about the relationships between serological responses and protection against omicron infection.
Omicron sublineages such as BA.1, BA.1.1, and BA.2 have contributed to the current wave of COVID-19 cases. In particular, BA.2 has become the dominant circulating strain in most of the world.
Taken together, the Omicron sublineages are distinguished by mutations in the antibody target spike protein. However, in vitro neutralization studies reveal equivalent vaccine-induced neutralizing potential against BA.1 and BA.2.
In a recent study published on the preprint server medRxiv*, emerging infections in healthcare workers (HCW) who received the booster vaccination either with or without prior SARS-CoV-2 infection were examined. During the study period, BA.1, BA.1.1, and BA.2 circulated in Stockholm, Sweden, thus permitting an analysis of breakthrough infections with the three sublineages. The researchers looked at serological correlates of protection against infection, symptoms, and viral ribonucleic acid (RNA) trajectories.
About the study
Between April and May 2020, 2,149 HCW enrolled in the COMMUNITY trial at Danderyd Hospital in Stockholm, Sweden. Since their involvement in the study, study participants were monitored every four months.
Seroconversion at any of the follow-up visits and/or polymerase chain reaction (PCR) results were used to confirm SARS-CoV-2 infection prior to immunization. Starting in January 2021, all HCW were immunized with either BNT162b2 (BNT) or ChAdOx1 nCoV-19 (ChAd) vaccines, depending on availability. All study participants had received the Moderna (MOD) mRNA-1273 vaccine as their third booster dose.
Serological responses following mRNA-1273 booster vaccination
In a prior investigation, the authors found that two doses of BNT or heterologous ChAd and BNT resulted in higher cross-neutralization potency against SARS-CoV-2 variants as compared to two doses of ChAd.
In the current study, the authors discovered similar booster anti-wildtype (WT) spike immunoglobulin G (IgG), anti-WT receptor-binding domain (RBD) IgG, cross-reactive IgG capable of binding to both the BA.1 spike and RBD, as well as neutralizing titers against both WT and BA.1 in blood samples obtained before and five weeks after receiving the Moderna mRNA-1273 booster vaccination.
After the initial vaccination, COVID-19-recovered vaccinees had higher antibody responses than SARS-CoV-2 naive vaccinees. The difference in cross-reactive IgG capable of binding to Omicron was maintained after the booster MOD vaccination.
Impact of booster vaccination on viral load and clearance
The researchers also compared antibody titers in vaccinated individuals who later tested positive for COVID-19 to those who remained negative throughout the screening period. The aim of these experiments was to determine whether those with breakthrough infections had reduced antibody responses prior to infection.
In individuals with and without later breakthrough infection, post-booster anti-WT spike IgG titers and neutralizing titers against both WT and BA.1 were identical. Comparatively, high titers of binding and neutralizing antibodies were associated with lower peak viral RNA levels and faster viral clearance.
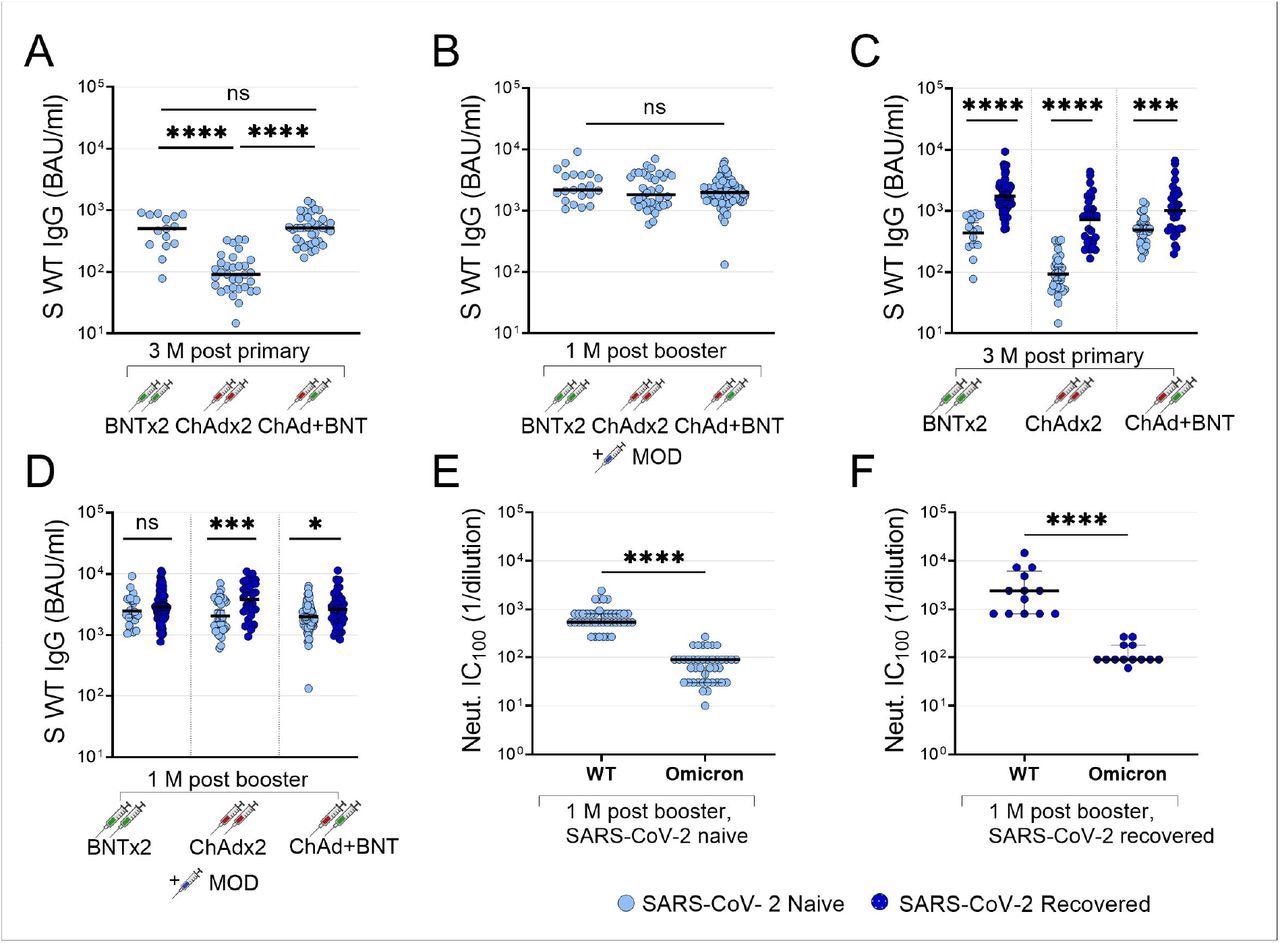
Impact of primary vaccine regimens and prior infection on booster antibody responses. Anti-WT spike IgG three months after primary vaccination series with two doses BNT, two doses ChAd or one dose ChAd followed by one dose BNT (A), and anti-spike IgG one month after MOD booster vaccine dose in the same participants (B). Anti-WT spike IgG in SARS-CoV-2 naïve (light blue dots) and recovered (dark blue dots) participants 3 months after primary vaccination series with two doses BNT, two doses ChAd or one dose ChAd followed by one dose BNT (C) and one month after MOD booster vaccine dose in the same participants (D). Microneutralizing titers against SARS-CoV-2 WT and omicron BA.1 in SARS-CoV-2 naïve (E) and recovered (F) participants one month after MOD booster vaccine dose. IgG titers are presented as binding antibody units (BAU)/ml and microneutralizing titers are presented as the lowest neutralizing dilution (1/Y). Lines depict geometric mean titers and bars depict a 95% confidence interval. S; spike, WT; wild-type, Neut; microneutralizing titer, BAU; binding antibody units; BNT; BNT162b2 mRNA vaccine, ChAd; ChAdox1 n-CoV 19 vaccine, MOD; mRNA-1273 vaccine, M; months, ns; P > 0.05, *; P ≤ 0.05, **; P ≤ 0.01, ***; P ≤ 0.001, ****; P ≤ 0.0001.
The association between viral RNA and symptoms
Common cold symptoms were commonly reported among the 55 individuals with symptomatic infection. When compared to the viral load in samples obtained from asymptomatic participants, the presence of symptoms at the time of sampling was associated with a greater viral load.
An increased viral load was also associated with fever, cough, headache, and anosmia that were reported at any point during infection.
Differences in Omicron sublineage breakthrough infections
As compared to neutralizing antibodies against the WT SARS-CoV-2, post-booster cross-reactive antibody responses that could neutralize Omicron BA.1 were significantly lower. Furthermore, cross-reactive antibody responses capable of neutralizing Omicron BA.2 were significantly greater than those capable of neutralizing BA.1.
Whole-genome sequencing (WGS) identified 26 BA.1, 21 BA.1.1, and 24 BA.2 infections in 71 out of 72 identified cases, with at least one sample exceeding a cycle threshold (Ct) value of 35. Regardless of the infecting Omicron sublineage, pre-infection anti-WT spike IgG levels were comparable in subjects who experience a breakthrough infection and those who remained negative for COVID-19.
The median Ct value of the initial positive sample in BA.1 and BA.2 infections was 29.4 and 25, respectively, thereby indicating a 100-fold higher quantity of viral RNA in BA.2 at an early stage of their infection; however, these observations were not significant. BA.2 breakthrough infections were also associated with longer clearance times as compared to BA.1 infections. Furthermore, symptom duration in BA.2 infected people was greater than in BA.1 infected people.
Implications
The kinetics responsible for SARS-CoV-2 Omicron shedding in immunized individuals, combined with information on possible immunological correlates of protection from infection, is important for the development of adequate infection control methods and vaccination strategies.
The findings from the current reveal a significant rate of Omicron breakthrough infections in HCWs who had recently received their third booster COVID-19 vaccine dose. It can therefore be inferred that the increased viral load associated with these breakthrough infections has contributed to the global rise in COVID-19 cases. Importantly, these findings also demonstrate that vaccine-induced antibody titers have a minor impact in predicting the likelihood of Omicron breakthrough infection.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Marking, U., Havervall, S., Norin, N. G., et al. (2022). High rate of BA.1, BA.1.1 and BA.2 infection in triple vaccinated. medRxiv. doi:10.1101/2022.04.02.22273333. https://www.medrxiv.org/content/10.1101/2022.04.02.22273333v1
- Peer reviewed and published scientific report.
Marking, Ulrika, Sebastian Havervall, Nina Greilert Norin, Oscar Bladh, Wanda Christ, Max Gordon, Henry Ng, et al. 2023. “Correlates of Protection and Viral Load Trajectories in Omicron Breakthrough Infections in Triple Vaccinated Healthcare Workers.” Nature Communications 14 (1): 1577. https://doi.org/10.1038/s41467-023-36984-1. https://www.nature.com/articles/s41467-023-36984-1.