In a recent study under review at the Scientific Reports journal and posted to the Research Square* preprint server, researchers assessed the impact of green tea catechin epigallocatechin gallate (EGCG) on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Coronavirus disease 2019 (COVID-19) vaccines have played a critical role in curbing the morbidity and mortality caused by SARS-CoV-2 infections. However, with the emergence of novel SARS-CoV-2 variants, there is an urgent need for effective therapeutic measures.
 Study: The green tea catechin EGCG provides proof-of-concept for a pan-coronavirus attachment inhibitor. Image Credit: jazz3311 / Shutterstock
Study: The green tea catechin EGCG provides proof-of-concept for a pan-coronavirus attachment inhibitor. Image Credit: jazz3311 / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
About the study
In the present study, researchers estimated and characterized the ability of EGCG to prevent infections of human seasonal and pathogenic CoVs, and bat and murine COVs.
The team examined the effect of EGCG on the infectivity of seasonal human CoVs (HCoV), including an alphacoronavirus called HCoV-229E and a betacoronavirus called HCoV-OC43. They treated HCoV-229E and HCoV-OC43 virions with dimethyl sulfoxide (DMSO) or different concentrations of EGCG, which were subsequently used for the inoculation of human hepatoma (Huh7) or human lung epithelial (A549) cell monolayers. Infectivity was later measured via plaquing efficiency on Huh7 cells or immunofluorescence focus-forming assay on Huh7 and A549 cells.
The researchers evaluated the antiviral activity of EGCG against betacoronaviruses by producing lentiviral particles that were pseudotyped with SARS-CoV-1, SARS-CoV-2, and Middle East respiratory syndrome (MERS)-CoV spike (S) proteins. The team also produced lentiviral particles pseudotyped with the S protein of WIV1-CoV, a bat coronavirus that binds to the human angiotensin-converting enzyme-2 (ACE-2).
The team subsequently assessed the influence of EGCG on replication-competent vesicular stomatitis virus (VSV) virions that expressed the green fluorescent protein (GFP) and the SARS-CoV-2 S protein instead of the VSV-SARS-CoV-2 glycoprotein. This analysis was conducted by infecting Huh7, A549- ACE2, and lung epithelial Calu-3 cells with VSV-SARS-CoV-2 virions treated with EGCG. The infected cells were later imaged under fluorescence microscopy.
The action of EGCG on CoV virions was confirmed by conducting time-of-addition experiments to understand the mechanism of pan-CoV activity by EGCG. Subsequently, binding assays were performed to test whether EGCG inhibited the attachment of CoV to cell surfaces. Furthermore, the researchers assessed whether heparin could inhibit HCoV-OC43 infection by treating the HCoV-OC43 virions with rising heparin concentrations.
Results
The study results showed that EGCG efficiently inhibited infectivity of both HCoV-229E and HCoV-OC43 at sub- to low micromolar concentrations. This observation was comparable to other viruses susceptible to EGCG inhibition, such as herpes simplex virus-1 (HSV-1). The team also observed that mouse hepatitis virus A-59 (MHV-A59) was inhibited by EGCG with a potency that was similar to that against the endemic human CoVs. Furthermore, EGCG had minimal impact on cell viability at low micromolar concentrations in which EGCG had antiviral activity. Overall, these observations suggested that EGCG targeted a highly conserved step viral infection.
The team found that EGCG sufficiently inhibited the pseudoparticle entry of SARS-CoV-2, SARS-CoV-1, MERS-CoV, as well as WIV1-CoV at the half-maximal inhibitory concentration (IC50). EGCG also inhibited the pseudoparticle entry of SARS-CoV-2, SARS-CoV-1, and WIV1-CoV into A549-ACE2 cells at IC50, and lentiviral particles pseudotyped with the SARS-CoV-2 Delta and Omicron variant S proteins.
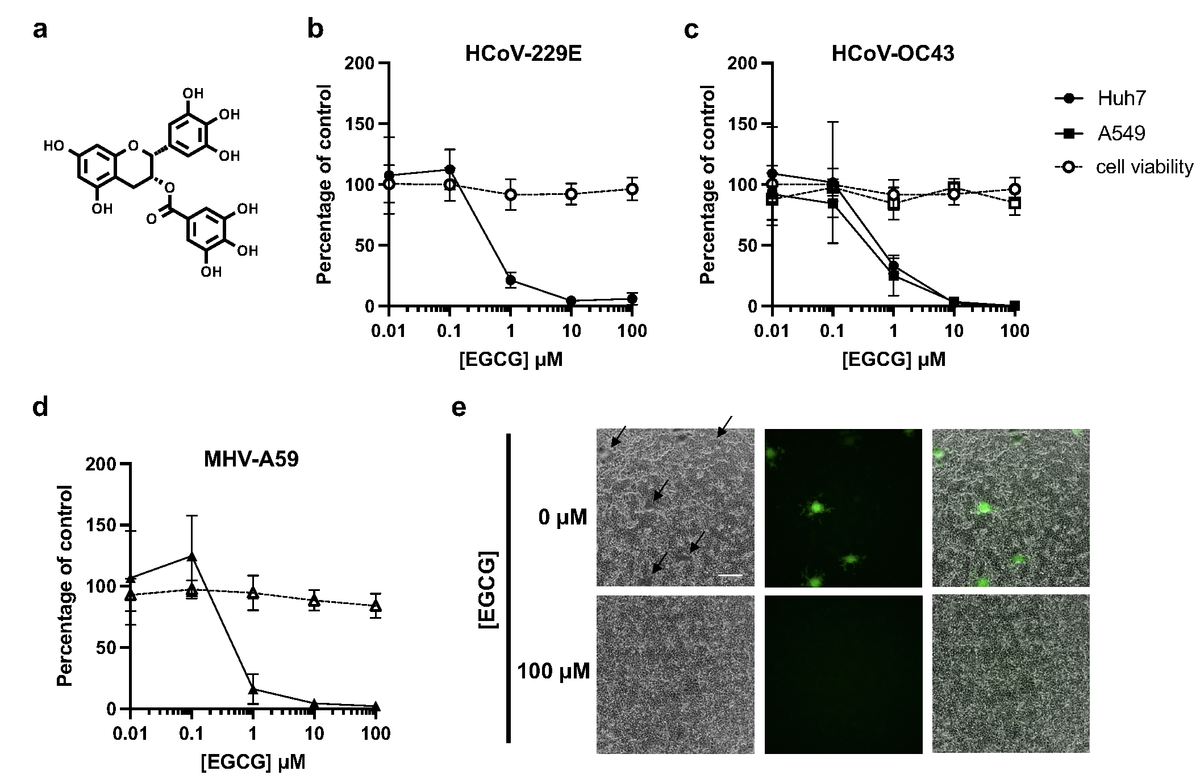
EGCG pre-treatment inhibits infection by diverse CoVs without affecting cell viability. (a) Structure of epigallocatechin gallate (EGCG). (b-d) Pre-treatment of authentic HCoV-229E, HCoV-OC43 or murine coronavirus MHV-A59 virions with EGCG for 10 min at 37°C inhibits infection of Huh7 (b,c), A549 (c) or L929 cells (d), respectively. Mean values with standard deviation from three independent experiments are plotted. Cell exposure to EGCG during the 2 h infection had minimal effect on cell viability (dashed line), where mean values with standard deviation of two independent experiments with duplicates are plotted. (e) A reduction in syncytia (black arrows) was observed for EGCG-treated MHV-A59. Scale bar: 200 mm.
Furthermore, VSV-SARS-CoV-2 infection was inhibited by EGCG at IC50 between 0.14 and 1.13 mM. These findings showed that the inhibitory activity of EGCG was dependent on the multiplicity of infection (MOI). Altogether, it indicated that EGCG inhibited infectivity of different CoVs, including human seasonal CoVs, pathogenic CoVs, and pre-emergent bat and rodent CoVs.
The team observed EGCG-elicited inhibition of infectivity in the time-of-addition experiments performed until the highest concentration of EGCG was achieved. However, this inhibitory activity was observed when the virions were already exposed to EGCG; this indicated that EGCG performed its inhibitory activity early in the infection process.
Furthermore, it was observed that EGCG reduced the binding of HCoV-OC43 and VSV-SARS-CoV-2 to Huh7 and Calu-3 cells to similar extents, despite the differences in the viral receptors involved. The team also found that heparin partially inhibited VSV-SARS-CoV-2 binding; however, heparin reduced the binding of HCoV-OC43. This suggested that glycosaminoglycans are involved in the entry of HCoV-OC43.
Moreover, researchers noted that inhibition of HCoV-OC43 depended on the rising concentrations of heparin doses. Overall, these findings indicated that heparan sulfate has an important role in the entry of endemic CoVs, and suggested that the inhibitory effect of EGCG was due to the inhibition of CoV attachment to heparan sulfate on the cell surface.
Altogether, the study findings show that EGCG exerted pan-CoV activity by disrupting the attachment of CoV with heparan sulfate. The researchers believe that the present study can form the foundation for developing therapeutics such as entry inhibitors that protect against novel emerging CoV variants.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources