A recent study published in the Centers for Disease Control and Prevention (CDC) Morbidity and Mortality Weekly Report (MMWR) assessed the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection-induced viral antibodies (Abs) seroprevalence in the United States (US).
 Study: Seroprevalence of Infection-Induced SARS-CoV-2 Antibodies — United States, September 2021–February 2022. Image Credit: vitstudio / Shutterstock
Study: Seroprevalence of Infection-Induced SARS-CoV-2 Antibodies — United States, September 2021–February 2022. Image Credit: vitstudio / Shutterstock
Background
The Omicron (B.1.1.529) variant of SARS-CoV-2, the causative agent of coronavirus disease 2019 (COVID-19), became the most common SARS-CoV-2 variant in the US in December 2021. As a result, COVID-19 case rates in the US reached new highs. Since some COVID-19 cases are asymptomatic, undiagnosed, or unreported, traditional disease surveillance techniques do not capture all SARS-CoV-2 cases. Therefore, SASR-CoV-2 seroprevalence assessment, i.e., the percentage of people with SARS-CoV-2 antibodies (Abs), can help researchers better understand the COVID-19 population-level incidence.
About the study
The present paper explored age group-based changes in COVID-19-induced SARS-CoV-2 Abs seroprevalence in the US from September 2021 to February 2022. The team used data from the 2018 American community survey and the CDC's national commercial laboratory seroprevalence investigation. The national commercial laboratory seroprevalence analysis was a recurrent, cross-sectional nationwide survey that assesses the percentage of the population with SARS-CoV-2 infection-induced Abs in 50 US states, the District of Puerto Rico, and Columbia.
Anti-nucleocapsid antibodies (anti-N Ab), generated in response to SARS-CoV-2 infection but not in reaction to the presently permitted COVID-19 vaccines, were examined in the collected sera samples. In addition, a convenience blood specimens sample presented for clinical assessment was tested for anti-N Abs every four weeks from September 2021 to February 2022. In February 2022, the sampling timeframe in 18 among the 52 jurisdictions was <2 weeks, while specimens from two jurisdictions were unavailable. To reduce selection bias, specimens for which SARS-CoV-2 antibody testing was ordered by clinicians were excluded.
The investigators calculated the seroprevalence rates over four weeks by age group (≥65, 50–64, 18–49, 12–17, and 0–11 years). Authors used raking spanning age, gender, and metropolitan status characteristics from the 2018 American community survey data to weight jurisdiction-level findings to the population to obtain estimates. Confidence intervals (CIs) were generated using bootstrap resampling, and nonoverlapping CIs were used to assess statistical differences. The Roche Elecsys anti-SARS-CoV-2 pan-immunoglobulin (Ig) immunoassay was used to examine all specimens. Further, R statistics software was used for all statistical analyses.
Results
The study results showed that the median sample size for each four-week term from September 2021 to January 2022 was 73,869 and the sample size for February 2022 was 45,810. Overall, SARS-CoV-2 seroprevalence rose by 0.9 to 1.9 percentage points every four weeks from September to December 2021.
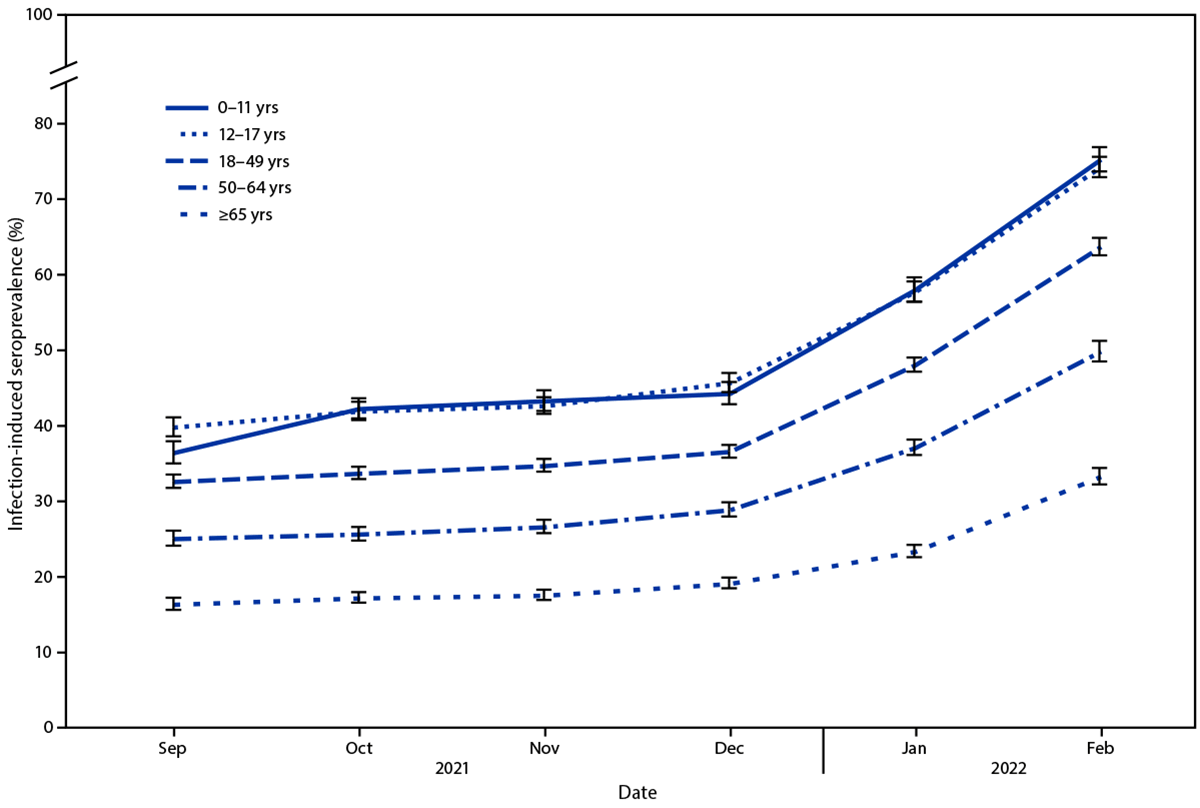
Seroprevalence of infection-induced SARS-CoV-2 antibodies, by age group — United States, September 2021–February 2022
Moreover, overall COVID-19 seroprevalence in the US rose from 33.5% to 57.7% between December 2021 and February 2022. During the same timestamp, seroprevalence among kids aged 0–11 years went from 44.2% to 75.2%, and among children aged 12–17 years increased from 45.6% to 74.2%. Seroprevalence hiked from 36.5% to 63.7% in individuals aged 18–49 years, 28.8% to 49.8% in adults aged 50–64 years, and 19.1% to 33.2% in adults aged ≥65 years.
As of February 2022, nearly 75% of adolescents and children had serologic evidence of prior COVID-19. In addition, around one-third of this population became newly seropositive after December 2021. From September 2021 to February 2022, the age groups with the least COVID-19 vaccination coverage experienced the highest increases in SARS-CoV-2 seroprevalence. Besides, by April 2022, the percentage of the US population that was fully vaccinated against COVID-19 increased with age (≥65 years, 90%; 50–64, 80%; 18–49, 69%; 12–17, 59%; and 5–11, 28%). Lower SARS-CoV-2 seroprevalence among individuals aged 65 years and older, at higher risk of severe COVID-19, might be linked to increased extra precautions usage among senior citizens.
Conclusions
According to the study, the SARS-CoV-2 Omicron variant had a high infection incidence, especially among children in the US. As the authors noted, seropositivity to anti-SARS-CoV-2 N Ab antibodies should not be construed as immunity to future COVID-19. COVID-19 vaccination remains the safest option for reducing SARS-CoV-2 infection sequelae in children and adults, including COVID-19-linked hospitalization. Following SARS-CoV-2 infection, COVID-19 vaccination offers extra protection against severe illness and hospitalization.
As a result of the present study, COVID-19 vaccination was significantly beneficial for all eligible individuals, including those who had already been exposed to SARS-CoV-2.