Automated AI review of coronary imaging pinpoints dangerous plaques more effectively than manual assessment, helping identify patients who need closer monitoring after a heart attack.
Artificial intelligence-based identification of thin-cap fibroatheromas and clinical outcomes: the PECTUS-AI study
In a recent study published in the European Heart Journal, a group of researchers compared artificial intelligence (AI)-based identification of thin-cap fibroatheroma (TCFA) on optical coherence tomography (OCT) with core laboratory (CL) review and related both to two-year patient outcomes in the pre-planned PECTUS-AI secondary analysis of the prospective PECTUS-obs cohort.
Background
Every minute, someone survives a heart attack and wonders if the next one is lurking; who is truly safe when arteries look open but plaques are unstable? Many events arise from TCFAs, lipid-rich plaques with fragile fibrous caps that rupture under blood-pressure surges. OCT provides micrometer-scale views of cap thickness; however, frame-by-frame reading is slow, inconsistent, and rarely performed across an entire vessel. AI promises standardized interpretation that scales to full pullbacks and reduces reader variability. Further research is needed to confirm whether automated detection has a meaningful impact on prognosis and decision-making.
About the study
This pre-planned secondary analysis used a prospective observational cohort of patients with myocardial infarction (MI). Operators imaged all intermediate, non-culprit lesions with a visually estimated stenosis of 30 to 90 percent and a fractional flow reserve (FFR) above 0.80 using OCT. A CL defined TCFA as a lipid plaque with a lipid arc ≥ 90 degrees and a minimum fibrous cap thickness < 65 micrometers at the frame level. An AI system, OCT-AID (an OCT-based AI segmentation tool), segmented pullbacks using a self-configuring nnU-Net version 2 model and quantified cap thickness and lipid content. To limit false positives, AI-identified TCFA required the criteria to be met in at least three of the ten consecutive frames.
A deep learning tool detected severe attenuation artifacts; frames were excluded if over 25% of A-lines were affected, and lesions or pullbacks with fewer than half of the frames analyzable were removed. The primary outcome was a two-year patient-level composite of death, non-fatal MI, or unplanned revascularization, excluding procedural and stent-related events and myocardial infarctions not clearly attributable to a specific segment; a committee adjudicated events. Time-to-event analyses employed the Kaplan-Meier method and log-rank test, with Cox models estimating the hazard ratio (HR) and 95% confidence interval (CI). Discrimination was assessed using the concordance statistic (C-statistic) and the DeLong test.
Study results
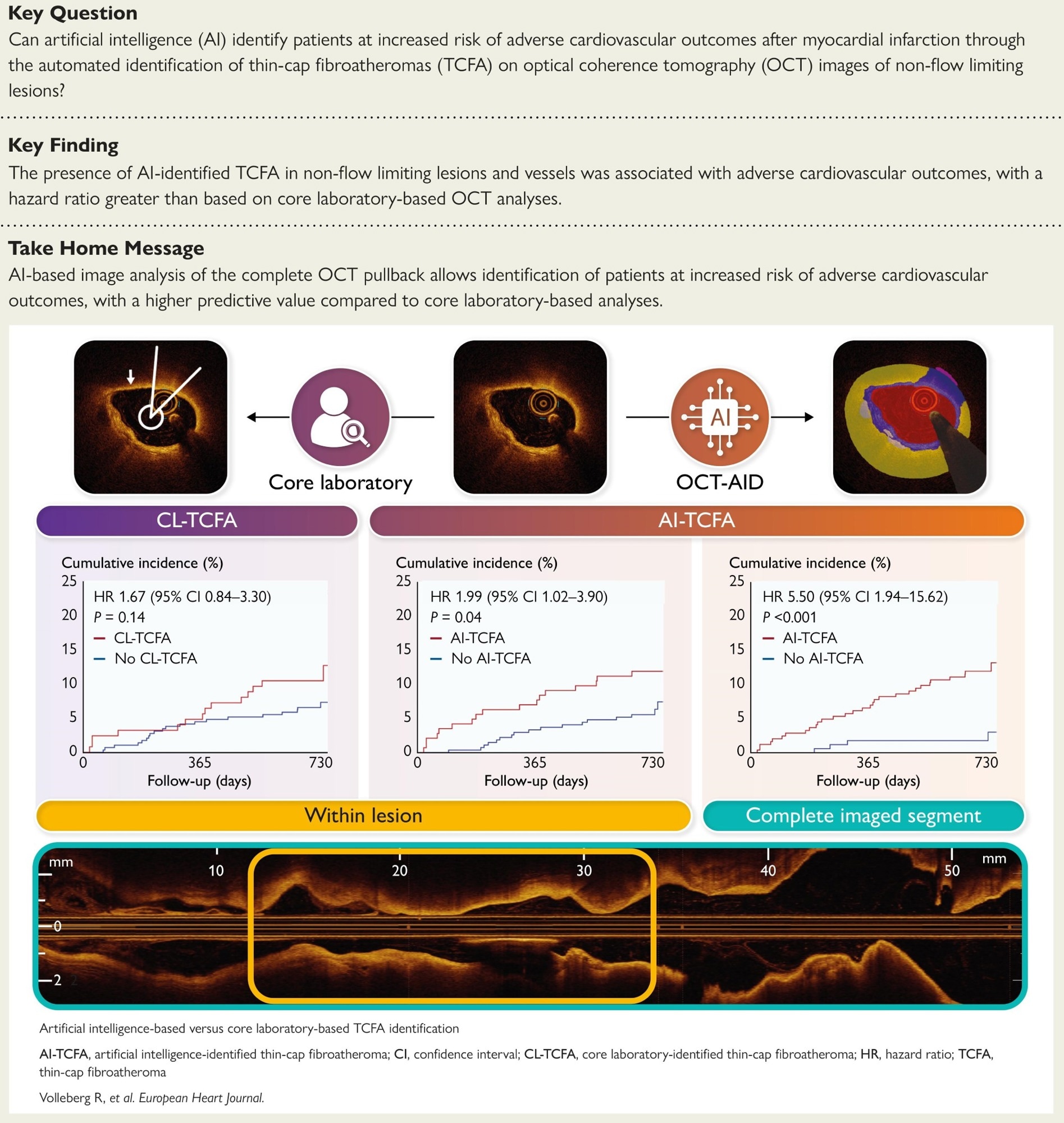
Among 414 patients (mean age, 63 years), hypertension and diabetes were present in 52.9% and 14.5%, respectively, and the presentation was split between ST-elevation myocardial infarction (STEMI, 51.4%) and non–ST–elevation myocardial infarction (NSTEMI, 48.6%). Across 488 target lesions, the left anterior descending artery (LAD) and Cx predominated; mean FFR was 0.89 ± 0.05. Artifact detection excluded a median of 0% of frames per lesion; six patients with over 50% non-analyzable frames were removed. Agreement between AI-identified TCFA and CL-TCFA was fair to moderate (κ ≈ 0.38 lesion-level; κ ≈ 0.40 patient-level); at least one AI-TCFA within target lesions occurred in 143 of 414 patients (34.5%) compared with 124 (30.0%) by the core laboratory.
Within target lesions, AI-TCFA identified a higher two-year risk than no AI-TCFA (11.9 vs 6.3%), for an HR of 1.99 (95% CI: 1.02 to 3.90; P = 0.04). CL-TCFA showed a nonsignificant association (11.3% vs 6.9%; HR: 1.67, 95% CI: 0.84-3.30; P = 0.14). Discrimination for the composite outcome was similar when limited to target lesions (C-statistic 0.58 for AI vs 0.56 for the CL; P = 0.65). At the complete-segment level, AI discrimination exceeded CL target-lesion assessment (0.66 vs 0.56; P = 0.03) and was numerically higher than AI target-lesion analysis (0.66 vs 0.58; P = 0.08).
Whole-segment analysis strengthened prognostic utility. In 411 patients with analyzable pullbacks, AI-TCFA was present anywhere in the imaged vessel in 243 (59.1%) and was associated with a higher risk of the primary outcome (12.3% vs 2.4%; HR: 5.50, 95% CI: 1.94 to 15.62; P < 0.001). Deaths were more frequent with AI-TCFA (5.3 vs 0.6%; P = 0.009) and unplanned revascularizations (7.4 vs 1.8%; P = 0.01); non-fatal MI was numerically higher (2.5 vs 0.6%; P = 0.14). The absence of AI-TCFA across the segment had a negative predictive value (NPV) of 97.6% (95% CI: 94.0-99.3) for excluding future events. A comprehensive AI review captured plaque vulnerability more effectively than lesion-focused human reading, identifying patients for tighter risk-factor control and surveillance.
Conclusions
AI applied to OCT standardized TCFA detection and identified patients at increased risk after MI. Compared with CL assessment of visually selected lesions, evaluating the complete imaged segment yielded stronger prognostic discrimination and a high NPV that can reassure low-risk patients while focusing attention on those at higher risk. Because frame-by-frame manual reading is time-consuming and variable, automated analysis could help translate high-resolution imaging into practical decisions about secondary prevention, surveillance, and focal therapy. Analyses were performed offline in a single observational dataset, and prospective on-site validation is still needed before routine adoption.
Journal reference:
- Volleberg, R. H. J. A., Luttikholt, T. J., van der Waerden, R. G. A., Cancian, P., van der Zande, J. L., Gu, X., Mol, J.-Q., Roleder, T., Prokop, M., Sánchez, C. I., van Ginneken, B., Išgum, I., Saitta, S., Thannhauser, J., & van Royen, N. (2025). Artificial intelligence-based identification of thin-cap fibroatheromas and clinical outcomes: the PECTUS-AI study. European Heart Journal. DOI: 10.1093/eurheartj/ehaf595, https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehaf595/8244402