New evidence reveals how everyday processed foods may disrupt gut barriers and amplify inflammation, while targeted dietary strategies offer promising ways to improve outcomes in Crohn’s disease.
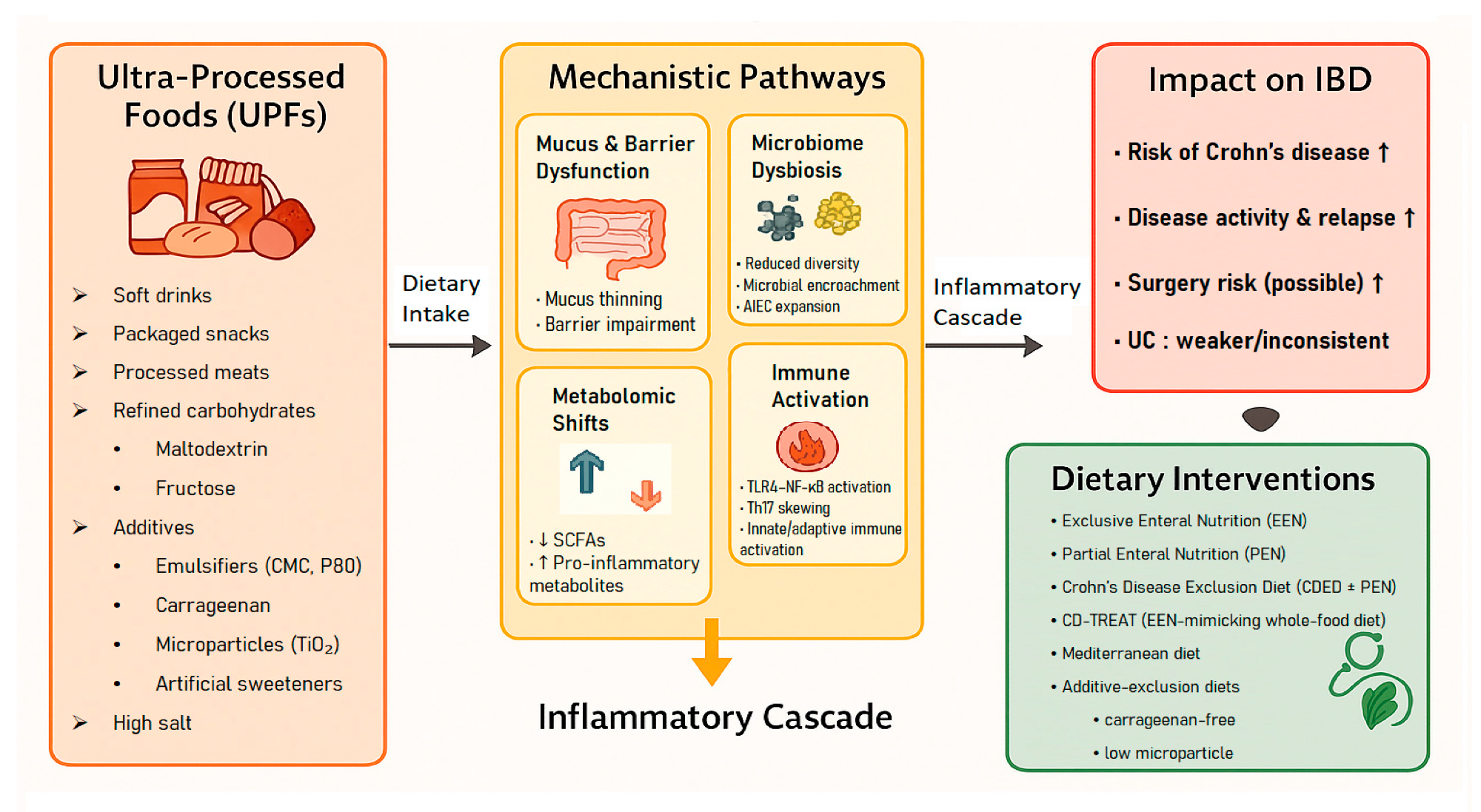
Conceptual framework linking ultra-processed foods (UPFs) to mechanistic pathways, inflammatory cascade, clinical outcomes, and dietary interventions in inflammatory bowel disease (IBD).
In a recent study published in the journal Nutrients, a group of researchers synthesized evidence linking ultra-processed foods (UPFs) to inflammatory bowel disease (IBD) risk, activity, mechanisms, and therapeutic dietary strategies.
Rising UPF Intake and Global IBD Trends
More than half of calories in many nations now come from UPFs, paralleling rising IBD, which includes Crohn’s disease (CD) and ulcerative colitis (UC), disorders that disrupt school, work, and family life.
The NOVA classification defines UPFs as industrial formulations rich in refined substrates and additives. Large cohorts link higher UPF intake with CD more than UC. Additives such as emulsifiers, carrageenan (CGN), and maltodextrin (MDX) can impair the intestinal barrier and microbiome, and many rely on packaged meals, hence guidance matters.
Further research is needed to standardize exposure, test mechanisms, and personalize nutrition, while also recognizing that prospective cohorts remain vulnerable to unmeasured confounding and early disease-driven dietary change.
Review Methods for UPF, IBD Evidence Synthesis
This narrative review was conducted with a structured approach to summarize epidemiological, mechanistic, and therapeutic evidence. Investigators searched PubMed/MEDLINE, Embase, and Scopus from January 2010 through March 2025 using combinations of terms for UPF, the NOVA, IBD, CD, and UC, and for specific additives such as emulsifiers (such as CMC), CGN, and MDX.
Additional mechanistic evidence on non-nutritive sweeteners (NNS), titanium dioxide (E171), and high salt derived from supplementary targeted searches rather than the core predefined strategy.
Reference lists of key studies and reviews were hand-searched. Human studies were eligible if they defined exposure by NOVA or additive doses and reported incident IBD, disease activity, relapse, or surgery.
Mechanistic models in animals or intestinal epithelial cells (IECs) were included when doses approximated dietary exposure, although many experiments still used concentrations higher than typical human intake.
Extraction captured exposure assessments (for example, food frequency questionnaire (FFQ)), outcome measures, and effect estimates including hazard ratio (HR), odds ratio (OR), and relative risk (RR).
Study quality, potential confounding by socioeconomic status (SES), and risks of reverse causation were qualitatively considered. Given heterogeneity across designs, populations, and outcomes, quantitative pooling was not attempted. Instead, evidence was synthesized narratively across three domains: epidemiology, mechanisms, and therapeutic implications, emphasizing recency, biological plausibility, and clinical relevance.
Outcomes were cross-checked for consistency in cohorts, while noting that NOVA classification inconsistency contributes to exposure misclassification.
UPF Intake and IBD Risk Across Cohorts
Across multinational cohorts, higher UPF intake was consistently associated with greater CD risk, with weaker or null findings for UC. In the Prospective Urban Rural Epidemiology cohort, five or more daily UPF servings were linked to incident IBD versus fewer than one (HR approximately 1.8).
Three large United States cohorts showed a dose-response association with CD, not UC. The NutriNet-Santé cohort reported no significant association, likely limited by few events and short follow-up. In the UK Biobank, the highest UPF intake predicted about a twofold higher CD risk (HR approximately 2.0) and a greater likelihood of IBD-related surgery.
Among patients, higher UPF intake correlated with active disease (OR approximately 3.8), and a remission cohort had more relapses over one year with the highest intake (HR approximately 3.9). Meta-analyses supported these signals, with a pooled RR of approximately 1.7 for CD and approximately 1.3 for overall IBD. However, the authors emphasize that these associations do not establish causality and require cautious interpretation, particularly because UPF consumption may correlate with broader lifestyle patterns that are difficult to fully adjust for.
Proposed Biological Pathways Linking UPFs to IBD
Mechanistic data provide plausibility. Emulsifiers such as CMC and P80 thin mucus, promote bacterial encroachment, reduce short-chain fatty acids (SCFAs), and shift the microbiota toward proinflammatory profiles, in a randomized controlled trial (RCT), CMC altered the microbiota, lowered fecal SCFAs, and increased abdominal discomfort. CGN activates toll-like receptor (TLR), Bcl10, and nuclear factor kappa B (NF-κB) signaling in IECs and accelerates relapse in UC in a double-blind RCT. MDX enhances adherence and biofilm formation by adherent-invasive Escherichia coli (AIEC), impairs antimicrobial defenses, and worsens colitis in interleukin-10, deficient mice.
NNS can impair human glucose tolerance via microbiome-dependent mechanisms. In models, E171 interacts with mucus and immune pathways; the European Food Safety Authority (EFSA) no longer considers E171 safe as a food additive. High-salt diets, common with UPFs, reduce Lactobacillus species, deplete SCFAs, and promote T helper 17 cell (Th17) inflammation.
The review notes that many mechanistic studies use high, continuous exposures that may not reflect typical human diets, and that translating these findings to clinical risk requires careful dose-response evaluation.
Dietary Strategies Targeting UPFs and Additives
Therapeutic evidence converges on reducing UPFs and selected additives. Exclusive enteral nutrition (EEN) remains first-line induction for pediatric CD and likely works partly by removing habitual exposures.
Partial enteral nutrition (PEN) is more acceptable but requires careful food selection. The Crohn’s Disease Exclusion Diet (CDED) plus PEN was non-inferior to EEN for induction and improved maintenance. Minimally processed patterns such as a Mediterranean-style diet or the Specific Carbohydrate Diet improve symptoms for some patients, though adherence and adult data vary.
Additive-focused approaches suggest benefits from CGN avoidance in UC and from low-microparticle diets targeting E171. The review emphasizes that evidence is strongest for pediatric CD, with adult and UC evidence still limited and heterogeneous, and that dietary strategies remain adjunctive rather than stand-alone therapy.
Clinical Implications for IBD Nutrition Guidance
Evidence from cohorts, mechanistic models, and trials indicates that UPF consumption is associated with higher CD risk and may worsen outcomes across IBD, with weaker signals for UC.
Additives, including CMC, P80, CGN, MDX, E171, and high salt, plausibly erode barrier function, shift the microbiome, diminish SCFAs, and activate TLR and NF-κB pathways and Th17 cell responses. Clinically, EEN, the CDED, and minimally processed patterns offer benefit.
Patients and families can prioritize meals while clinicians personalize plans, standardize tools, and support trials, but the authors note that professional guidelines currently prioritize whole-food dietary patterns rather than categorical UPF elimination because causal evidence is still emerging.
Journal reference:
- Choi, S. Y., & Moon, W. (2025). Ultra-Processed Foods and Inflammatory Bowel Disease: A Narrative Review of Epidemiology, Mechanisms, and Dietary Implications. Nutrients. 17(24). DOI: 10.3390/nu17243852, https://www.mdpi.com/2072-6643/17/24/3852