The COVID-19 pandemic has caused a pressing need for the rapid development of both preventive and therapeutic approaches to fight the virus. Given the urgency, going through the traditional drug discovery and development pathway is not feasible. This is where approaches like drug repurposing or repositioning become attractive. Drug repurposing is nothing but identifying new indications for existing, clinically approved drugs.
Although several existing drugs are being tested in various clinical studies to determine their efficacy in fighting COVID-19, so far, the only drug to achieve significant survival benefit is dexamethasone. Another example of drug repurposing against COVID-19 is chloroquine/hydroxychloroquine, an anti-malarial drug that has shown some activity against coronaviruses during previous pandemics. Hydroxychloroquine is also used as an anti-inflammatory drug in the treatment of autoimmune disorders. However, several well-designed clinical studies have proved that hydroxychloroquine is ineffective in the prevention or treatment of COVID-19 in patients with different levels of disease severity.
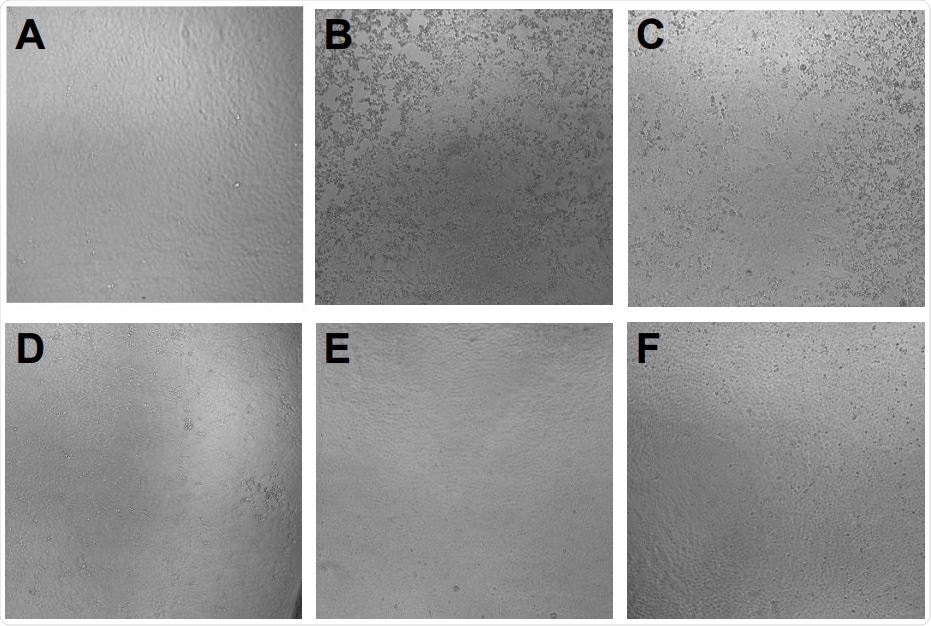
Prevention of SARS-CoV-2 induced cytopathic effect by azelastine. Vero E6 cells were infected with SARS-CoV-2 simultaneously with the addition of 0·4 to 25 µM of azelastine and continued to be cultured without the virus in the presence of the respective concentrations of the drugs. Cytopathic effect was assessed by light field microscopic examination of cultures 48 hours postinfection. A: uninfected (negative) control, B: virus infected (positive) control, C: virus + 3·125 µM azelastine, D: virus + 6·25 µM azelastine, E: virus + 12·5 µM azelastine, F: virus + 25 µM azelastine. G: Scoring system and summary of cytopathic effect in the presence of azelastine (results of two independent experiments).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The drug repurposing study
In this study, the team of researchers applied a novel computational prediction approach that is based on biochemical pathways. Their strategy was based on the poly-pharmacological hypothesis that says drugs interact and interfere simultaneously with several different targets and thus rewire the biochemical pathway networks.
Drug identification thus involves finding a drug that matches a pre-defined pathway modulation profile. The starting point of the team’s approach is a description of the inherent chemical properties of small drug molecules, with proven activity against the SARS-CoV-2 virus and implications for specificity to protein targets.
Using this unique descriptor, the team set off to identify unexpected or hidden drug similarities and identify new protein targets in the underlying biochemical pathways.
“Key to our strategy is the poly-pharmacological hypothesis, that drugs simultaneously interact and interfere with numerous targets and thereby rewire the biochemical pathway networks,” says the team.
The researchers tested the antiviral activity of the most predicted drug, azelastine in vitro in SARS-CoV-2 infection assays using reconstituted human nasal tissue and Vero E6 monkey kidney epithelial cells. The effect of this drug on viral replication was determined by viral genome quantification using digital droplet polymerase chain reaction (PCR).
What did they find?
The computational approach helped them identify drugs and drug families, some with proven activity and clinical efficacy against SARS-CoV-2. Azelastine, an histamine 1 receptor-blocker, was predicted by the approach in multiple screens. Due to its attractive safety profile and easy availability in a nasal formulation, it was chosen for experimental testing.
The results showed that azelastine significantly reduced cytopathic effect and inhibited SARS-CoV-2 infection of Vero E6 cells both in preventive and treatment settings. They also tested a 5-fold dilution of azelastine in a commercially available nasal spray. They found that it was very potent in inhibiting the propagation of the SARS-CoV-2 virus in infected human nasal tissue. The team concluded that the antihistamine azelastine might be considered for use in topical prevention or treatment of SARS-CoV-2 nasal colonization.
According to the team, azelastine, as such, could be helpful in reducing viral transmission and prophylaxis of COVID-19. However, the potential benefits of the drug needs to be proven in further clinical studies.
They also noticed a significant overlap among the three predicted and the experimental KEGG pathways, the most prominent between the SSAA09E2 and hydroxychloroquine pathways. The most common disease areas associated with the predicted pathways were viral infections including hepatitis C, measles, and influenza; parasitic infections such as Leishmaniasis, Trypanosomiasis, malaria; and bacterial infections such as tuberculosis, Salmonella, Shigella, and Pertussis.
“Interestingly, we found some overlap with drugs predicted by a complex network approach reported by the Barabási group, which relies on information about human protein binding partners where potential drug candidates are likely to perturb the interactome network relevant for viral infection.”
Several of the drugs that this research predicted are currently being tested in various clinical studies. Of note, dexamethasone has been shown to reduce mortality by one third in COVID-19 patients on mechanical ventilation. Two other drugs, famotidine, an H2-blocker antihistamine that decreases gastric acid production, and telmisartan, an anti-hypertensive drug, have been recently shown to improve morbidity in hospitalized SARS-CoV-2 infected patients.
“We argue this to be a more promising approach compared to conventional single-target drug design strategies, particularly in view of the multi-factorial disease phenotype of COVID-19.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
The Anti-histamine Azelastine, Identified by Computational Drug Repurposing, Inhibits SARS-CoV-2 Infection in Reconstituted Human Nasal Tissue In Vitro Robert Konrat, Henrietta Papp, Valeria Szijarto, Tanja Gesell, Gabor Nagy, Monika Madai, Safia Zeghbib, Anett Kuczmog, Zsofia Lanszki, Zsuzsanna Helyes, Gabor Kemenesi, Ferenc Jakab, Eszter Nagy, https://www.biorxiv.org/content/10.1101/2020.09.15.296228v1
- Peer reviewed and published scientific report.
Konrat, Robert, Henrietta Papp, Janine Kimpel, Annika Rössler, Valéria Szijártó, Gábor Nagy, Mónika Madai, et al. 2022. “The Anti-Histamine Azelastine, Identified by Computational Drug Repurposing, Inhibits Infection by Major Variants of SARS-CoV-2 in Cell Cultures and Reconstituted Human Nasal Tissue.” Frontiers in Pharmacology 13 (June). https://doi.org/10.3389/fphar.2022.861295. https://www.frontiersin.org/articles/10.3389/fphar.2022.861295/full.