The COVID-19 pandemic has still not been countered by effective drugs or antivirals, which is the reason for the continued restrictions on mobility and social interactions in most countries the world over. Now, a new study by researchers from Pfizer, University of Arizona, Purdue University, The Scripps Research Institute, and UC San Diego School of Medicine and published on the preprint server bioRxiv* in September 2020 reports that an enzyme inhibitor may act in synergism with the approved antiviral remdesivir to suppress viral replication in COVID-19.
Remdesivir is an RNA-dependent RNA polymerase (RdRp) inhibitor that has received emergency use authorization by the US Food and Drug Administration (FDA) in May 2020 for the treatment of SARS-CoV-2. Remdesivir trials have revealed that this drug reduces the time to recovery for COVID-19 patients, but not the severity of the disease. To enhance its clinical impact, it is necessary to find other drugs that may add to its therapeutic efficacy.
About PF-00835231
The drug candidate PF-00835231 discussed in the current study acts on the SARS-CoV-2 main protease or 3C-like protease (3CL protease or 3CLpro). This is required for viral replication since it causes the large viral polyprotein p1a/p1ab to be cleaved at 10 or more junctions. The result is the production of several non-structural proteins that play a crucial part in viral replication and transcription. These include RdRp, the helicase, and the 3CLpro itself.
Also, viral 3CLpro has no comparable human analogs. These two properties make it an ideal candidate for further investigation as a potential COVID-19 therapeutic.
Protease inhibitors have played a similar role in HIV and HCV therapeutics. Following this lead, the current researchers identified a small molecule protease inhibitor PF-00835231 that was active against SARS-CoV, the virus responsible for the earlier respiratory illness outbreak of 2002.
Produced by structure-based drug design, this drug never saw the light of day because of the rapid termination of the outbreak by stringent public health measures. Now, the researchers are taking a fresh look at it because of the high degree of identity between the SARS-CoV and SARS-CoV-2 3CLpro. In fact, they have entirely identical active sites.
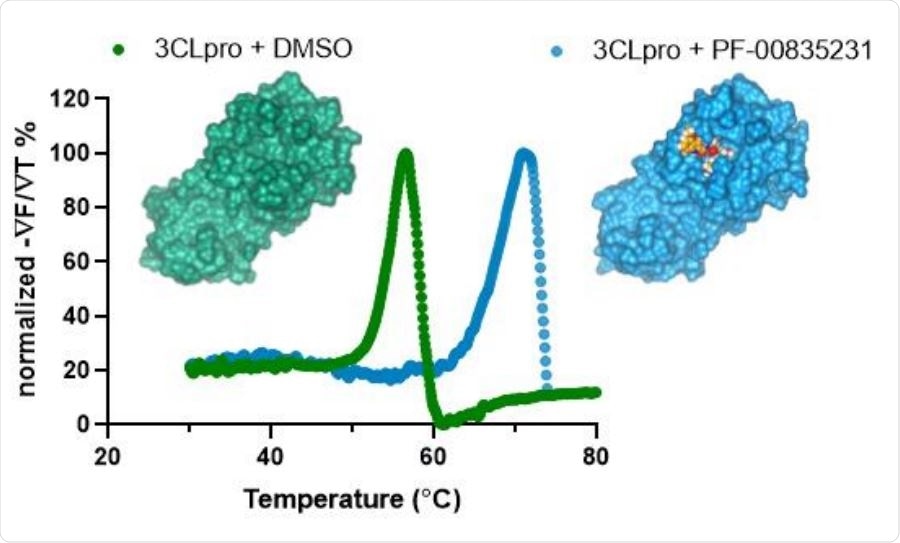
Representative thermal shift binding data of PF-00835231 with SARS-CoV-2 3CLpro. X-ray structures of SARS CoV-2 3CLpro apoenzyme (left) and SARS CoV-2 3CLpro in complex with PF-00835231 (right).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
PF-00835231 Binds CoV 3CLpro Selectively and Tightly in Vitro
The researchers found that both PF-00835231 and its phosphate prodrug has antiviral activity against a broad spectrum of coronaviruses (CoVs). It binds tightly to the enzyme at the catalytic cysteine residue, potently suppressing its activity. This enzyme being conserved across several CoVs. This allows it to inhibit several CoV 3CLpro enzymes in vitro, from alpha-, beta- and gamma-CoVs. However, it is inactive against human and HIV proteases, indicating its specificity of activity.
PF-00835231 has Anti- SARS-CoV-2 Cellular Activity in Vitro
The researchers examined the ability of PF-00835231 to exert antiviral activity against SARS-CoV-2 in cell culture, using a cytopathic effect (CPE) assay. As expected from experience with other viral protease inhibitors, they found that there was a dose-dependent but steep suppression of viral activity with this molecule.
When repeated using cell lines, which are more similar to the human lung cells, they obtained similarly promising results. Thus, this drug candidate has in vitro antiviral activity even as a single agent.
Combination of PF-00835231 with Remdesivir
When antiviral agents are combined, especially when they act on different steps of the replication cycle, the effects are often much more significant than when used alone. Evaluation of the combined activity of these drugs led to the finding that while the in vitro EC50 for PF-00835231 alone was 0.14 μM, and for remdesivir, it was 0.074μM, in combination, they showed a mix of synergistic and additive effects. This could be because different convalescent sera were used for the detection process.
Good Pharmacological Profile
The researchers also found that the novel drug candidate PF-00835231 is not easily metabolized, which reduces the chances of drug-drug interactions when used alongside other drugs. When tested for potential inhibition of transporter proteins, the researchers found that the risk was low at likely clinical concentrations.
Plasma protein binding of PF-00835231 is moderate, with 0.33 to 0.45 free fractions in plasma in several animal species. It is not well absorbed on oral administration because of its reluctance to dissolve, and low permeability, as well as the potential for it to be hydrolyzed by the gut enzymes. In monkey, rat, and dog experiments, only a tenth or less was eliminated intact in the urine. This shows that it is not largely excreted by the kidneys.
As a result, the in vivo and in vitro data are interpreted to mean that PF-00835231 could be used as an intravenous infusion for optimal efficacy. The researchers projected a minimally efficacious concentration (Ceff) that would achieve viral inhibition in clinical situations. They found that at this dose of PF-00835231 (by continuous infusion), there would be consistent anti-SARS-CoV-2 activity in a variety of cells. The study says, ‘Based on the human PK predictions, the minimally efficacious dose of PF-00835231 necessary to achieve this exposure is ~320 mg/day administered as a continuous intravenous infusion.” If this drug performs anything like remdesivir, it may take 10 days of administration to improve patient outcomes.
Encouraging Preclinical Safety Profile
The investigators also found that there is a wide safety margin between the effective and toxic concentrations of PF-00835231. This means that multiple of the effective dose can be tested in humans during clinical trials to understand the dose-response relationship safely and to ensure high levels of viral inhibition.
Again, because it does not show the potential for “overlapping or additive toxicity” with the drugs in current use for COVID-19 treatment, which means it can be combined with these agents with ease in humans.
Implications
The drug candidate PF-00835231 appears to be an active, highly selective, and potent CoV 3CLpro inhibitor over a broad spectrum of CoVs. At present, in vitro assays indicate intense antiviral activity over several cell types, including those which are similar to human lung tissue. The human pharmacokinetics of PF-00835231 is predicted to allow the drug to achieve effective unbound concentrations of 0.5μM, the estimated Ceff, if given at a dose of 500 mg by infusion over 24 hours in less than 350 mL. This can be scaled up in multiples in order to achieve efficacy if required.
Its safety profile, coupled with the robust antiviral and encouraging pharmacokinetic attributes, invite optimism about the results of its clinical trials either as a single-agent antiviral or along with other antivirals directed against other steps of the viral life cycle.
Overall, PF-07304814 exhibits an encouraging preclinical profile that has the SARS-CoV-2 antiviral activity, ADME, and safety profile that supports progression to the clinic as a potential novel COVID-19 single-agent antiviral treatment, with potential for further additional benefit in combination with antivirals that target other critical stages of the coronavirus life cycle. The favorable profile of PF-07304814 warrants clinical evaluation.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources