New insights into cellular aging, perfusion technologies, and senescence-targeting treatments show how aging organs could be revived, turning thousands of discarded donor organs into viable, lifesaving grafts.
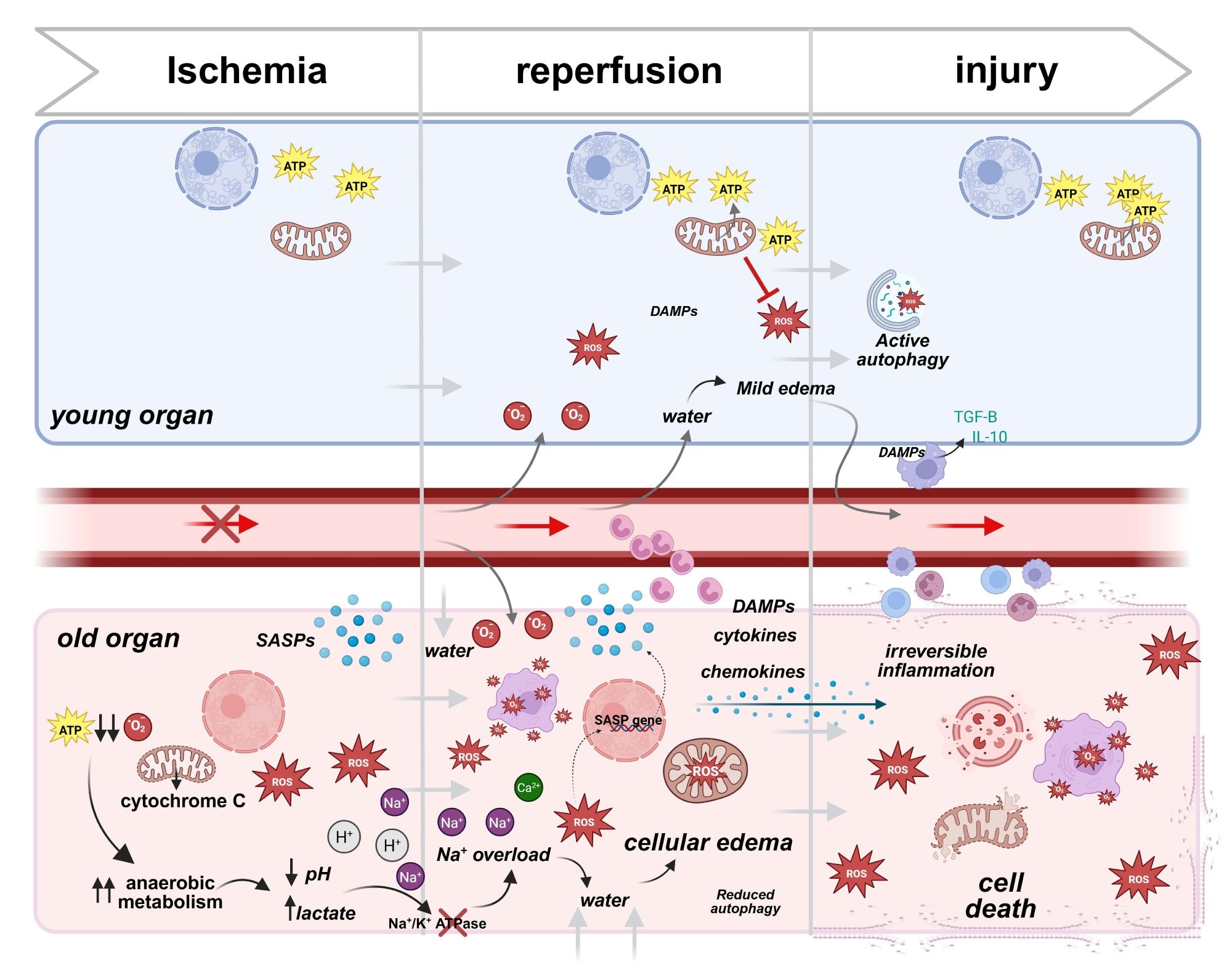
Cellular responses to ischemic reperfusion injury in young vs older donor organs. During ischemia, the deprivation of oxygen and nutrients leads to mitochondrial dysfunction and energy loss. In young cells, mitochondrial resilience helps sustain ATP production, ameliorating damage. Old cells, in contrast, experience significant ATP depletion, relying heavily on anaerobic metabolism, which leads to lactate buildup, pH reduction, and cellular stress. Upon reperfusion, the restoration of blood flow triggers oxidative stress as mitochondria generate excessive reactive oxygen species (ROS). Young cells compensate for those events through robust antioxidant systems, maintaining cellular integrity. Conversely, in old cells, impaired antioxidant defenses result in unregulated ROS production, furthermore damaging membranes, organelles, and DNA. Additionally, old cells release pro-inflammatory genes, amplifying local inflammation. Consequences are particularly severe in aged vascular endothelial cells, with ion pump dysfunction (e.g., Na⁺/K⁺ ATPase) causing ionic imbalances and cellular edema. This disruption exacerbates ischemic injury, progressing to irreversible damage. In contrast, young cells effectively resolve edema and inflammation through mechanisms that include macrophage945 mediated clearance of Damage-Associated Molecular Patterns (DAMPs) and anti-inflammatory cytokine release (e.g., IL-10 and TGF-β), allowing recovery and tissue repair. In old cells, persistent ROS generation, unresolved inflammation, and DAMP accumulation lead to irreversible inflammation, organelle collapse, and eventual cell death. Created in BioRender. Kayumov, M. (2025) https://BioRender.com/m23u7ro .
In a recent review published in the journal Nature Communications, researchers examined why organs from older donors are often discarded, highlighting emerging strategies aimed at rejuvenating these organs for safe transplantation. The review also emphasizes that most rejuvenation approaches remain preclinical and that safety considerations are critical before clinical adoption. They discussed technological and therapeutic advances that show strong potential to improve the viability of older donor organs and help address the organ shortage crisis.
Organ Shortage and Donor Age Challenges
Organ transplantation is essential for patients with end-stage organ failure, but demand overwhelmingly exceeds supply. In the United States alone, more than 104,000 people were waiting for an organ in late 2024, and around 7,000 die annually before receiving one. A major contributor to the shortage is the underutilization of organs from older donors. Despite the availability of thousands of potentially transplantable organs each year, nearly 40,000 (mostly from donors over 50) are discarded due to concerns about quality, functional decline, and higher risk of complications.
Older organs are more vulnerable to ischemia-reperfusion injury (IRI), show reduced physiological reserves, and have impaired repair capacity. These limitations make them more likely to fail early after transplantation, discouraging their use. Recognizing this, researchers are exploring new approaches to better preserve, modify, and rejuvenate aging organs, thereby safely expanding the donor pool. Importantly, the review underscores that organ-specific vulnerabilities vary, with the hearts and lungs exhibiting greater IRI sensitivity than the kidneys or livers. The authors also note that allocation policies and ethical considerations will need to evolve as organ-rejuvenation technologies advance.
Why Older Donor Organs Underperform
IRI is an unavoidable hurdle in transplantation. During ischemia, a reduced oxygen supply compromises mitochondrial adenosine triphosphate (ATP) production, leading to acidosis, ionic imbalance, and cell swelling. When blood flow is restored, a burst of reactive oxygen species generates oxidative stress, inflammation, and endothelial damage.
Older organs are particularly susceptible. Clinical studies show higher rates of primary graft dysfunction in livers, kidneys, hearts, and lungs from donors aged 50 and older. Mechanistically, aging reduces mitochondrial reserves, increases microvascular fragility, and lowers the expression of protective proteins that normally dampen inflammation. Older organs, therefore, sustain more structural damage and struggle to recover after IRI.
Aging also leads to structural and molecular changes that compromise organ function long before transplantation. A major hallmark is the accumulation of senescent cells, identifiable by markers such as SA-β-gal and p16^INK4a. These cells no longer divide or function normally and secrete pro-inflammatory molecules known as the senescence-associated secretory phenotype (SASP). SASP fuels fibrosis, disturbs tissue homeostasis, and promotes dysfunction.
Other contributors to age-related decline include vascular stiffening, reduced elasticity, and decreased blood flow, which drop by up to 50% in the liver by age 50. Higher blood viscosity and impaired hormonal signalling add further stress. The review notes that exposing older donor animals to young plasma improves outcomes primarily in liver IRI models, suggesting the systemic environment contributes significantly to functional decline.
Immune Activation and Rejection Risks
Older grafts provoke stronger immune responses and are more likely to be rejected, especially within the first year. The combination of IRI, SASP factors, and impaired anti-inflammatory capacity enhances the release of danger-associated molecular patterns (DAMPs), including circulating mitochondrial deoxyribonucleic acid (DNA). These signals intensify inflammation, attract immune cells, and heighten alloimmune activation. The review highlights biomarkers such as circulating mitochondrial DNA and interleukin-6 as emerging tools for assessing organ viability and injury severity.
Machine Perfusion and Preservation Advances
A key emphasis of the review is the central role of machine perfusion technologies. Normothermic and hypothermic machine perfusion allow ex vivo assessment of organ viability, targeted delivery of therapeutics, and metabolic recovery before transplantation. The authors also highlight subnormothermic storage at 10°C, which preserves mitochondrial function and reduces IRI across organ types.
Normothermic regional perfusion (NRP) is further identified as a promising technique for older donation-after-circulatory-death (DCD) organs, supporting in situ resuscitation and repair. The review proposes a two-phase rejuvenation model that combines a pre-transplant perfusion-based therapeutic cocktail with post-transplant regenerative support using cell-based therapies.
Senolytic Drug Strategies
Senolytics selectively eliminate senescent cells and reduce SASP-driven inflammation. The best-known combination, dasatinib and quercetin, targets anti-apoptotic pathways that senescent cells rely on. Preclinical studies demonstrate improved function in multiple tissues, including the heart, and reductions in circulating mitochondrial DNA. Other senolytics include fisetin and navitoclax, both of which induce apoptosis in senescent cells and have shown benefits in reducing fibrosis and hypertrophy. The review stresses that these data remain preclinical and that their safety in transplant recipients is not yet established.
Senomorphic and Metabolic Modulators
Conversely, senomorphics do not kill senescent cells but dampen the harmful effects of SASP. Metformin reduces SASP by lowering inflammation and oxidative stress. In liver perfusion and transplant models, metformin improves ATP production and reduces injury. Rapamycin, an immunosuppressant, prevents the accumulation of senescent cells and preserves graft structure. Resveratrol enhances mitochondrial function and reduces oxidative stress, with early evidence suggesting improved metabolic and inflammatory profiles. Ruxolitinib, a Janus kinase (JAK) inhibitor, reduces cytokine release and protects endothelial cells from injury.
The authors also describe mitochondrial-targeted antioxidants and metabolic cofactors, such as coenzyme Q10 and nicotinamide riboside, as promising agents that improve mitochondrial resilience in preclinical transplant models. The review additionally discusses compounds such as N-acetylcysteine and statins, which may modulate oxidative stress and inflammation during reperfusion, although results remain inconsistent.
Anti-Fibrotic and Regenerative Approaches
The review highlights anti-fibrotic or fibrinolytic compounds, including losartan, blebbistatin, nattokinase, and lumbrokinase, which may reduce established fibrosis in older organs. Stem-cell-based strategies, including mesenchymal stromal cells (MSCs) delivered during machine perfusion, show potential to reduce inflammation, enhance regeneration, and improve microvascular function in aged grafts.
A novel senescent-cell vaccine targeting the protein GPNMB is also discussed as an emerging, lower-toxicity alternative to traditional senolytics.
Anti-Inflammatory Interventions
Because aging-associated inflammation contributes strongly to graft dysfunction, anti-inflammatory drugs may help prepare older organs for transplantation. Corticosteroids are widely used in recipients, but experimental evidence indicates that treating donor organs before transplantation may also reduce inflammation and improve outcomes. Nonsteroidal anti-inflammatory drugs (NSAIDs), such as aspirin, may offer additional benefits by targeting certain inflammatory pathways, although evidence in aged organs remains limited.
Future Outlook for Organ Rejuvenation
The underuse of older donor organs significantly contributes to the global organ shortage. Aging increases susceptibility to IRI, functional decline, and immunogenicity, but new strategies, including senolytics, senomorphics, anti-inflammatory therapies, machine perfusion platforms, mitochondrial modulators, anti-fibrotic treatments, and stem-cell-based interventions, show strong potential to rejuvenate aging organs.
However, the review emphasizes that most interventions remain in early-stage or preclinical development and require rigorous safety and efficacy testing. Future progress will also depend on integrating biomarkers, refining allocation frameworks, and validating the proposed two-phase rejuvenation strategy in clinical trials. Continued research may substantially expand the donor pool and improve transplant outcomes, helping bridge the gap between organ supply and clinical need.
Journal reference:
- Kayumov, M., Song, Z., Martin, F., Tsou, S., Xiao, Y., Zhou, H., & Tullius, S. G. (2025). The promise of organ rejuvenation to overcome the shortage in organ transplantation. Nature Communications. DOI: 10.1038/s41467-025-66133-9, https://www.nature.com/articles/s41467-025-66133-9