The search for antivirals and vaccines capable of blocking the cell entry and infection produced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues as the colder season in the northern hemisphere approaches, threatening further waves of COVID-19.
The search includes immunological agents, enzyme inhibitors, and soluble competitive inhibitors. Now, a new study published on the preprint server bioRxiv* in October 2020 provides preliminary evidence that small molecules can inhibit viral entry by inhibiting spike-ACE2 attachment.
Finding Therapeutic Targets
The entry of SARS-CoV-2 into the host cell requires the interaction of the spike protein on the virus with the host cell receptor, angiotensin-converting enzyme (ACE2). This protein-protein interaction (PPI) presents a potential target for antiviral drugs.
The first avenue of PPI inhibition is by antibodies, but a more straightforward approach is by small-molecule inhibitors (SMIs), which are more robust to escape mutations and to strain variations. SMIs are also amenable to oral or inhaled administration, less likely to provoke an immune response, and easier to control.
Such attempts have been made after the SARS outbreak of 2002-2004, with many researchers conducting high-throughput screening (HTS) assays to identify drugs that could inhibit early viral entry. This included some SMIs. However, none of them have made it into clinical development. This is primarily due to their poor bioavailability, toxicity, and poor pharmacokinetics.
The researchers first screened their small molecule library to identify compounds like Congo red and Evans blue and novel compounds, which suppress the spike-ACE2 interaction at low micromolar levels. Such dyes are by default good protein binders, which indicates that their structures are so designed, providing a useful platform for the beginning of such attempts.
The dyes themselves are not typically therapeutically useful, being brightly colored and also prone to rapid degradation, in the case of azo dyes. However, they can be chemically modified to overcome these drawbacks.
Inhibition of SARS-CoV-2 Pseudovirus Entry
Based on this premise, the researchers used this approach to find potential ACE2-S interaction inhibitors among SMIs, which can be therapeutically developed. The proof that this is a valid approach comes from earlier research on other zoonotic coronaviruses.
The selected compounds prevented a pseudovirus expressing SARS-CoV-2-S protein from entering a cell bearing ACE2 receptors, with a half-maximal inhibitory concentration (IC50) at low micromolar levels. This could indicate the potential of such organic dyes to provide a chemical scaffold to identify novel PPI inhibitors.
The use of protein thermal shift assays showed that the identified SMIs bound specifically to the SARS-CoV-2-S protein and not to the ACE2 molecule. The researchers also found a common structural motif, in the presence of a biphenyl linker with two aromatic groups at the ends, one a naphthyl and the other an aromatic naphthyl or phenyl, and with one or more polar substitution groups.
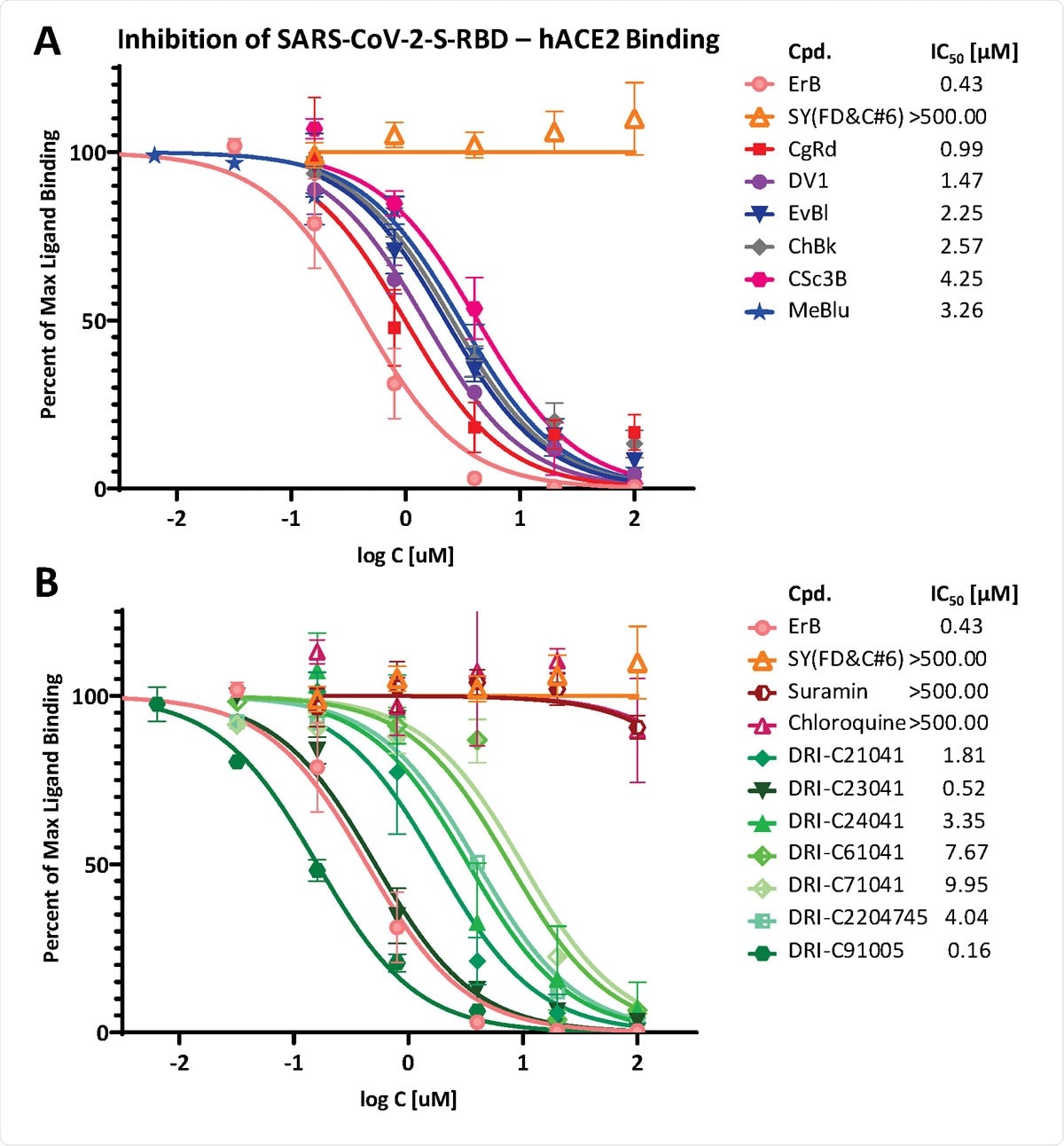
Concentration-dependent inhibition of SARS-CoV-2-S-RBD binding to ACE2 by compounds of the present study. Concentration-response curves obtained for the inhibition of the PPI between SARS-CoV-2-RBD (His-tagged, 0.5 μg/mL) and hACE2 (Fc-conjugated, 1 μg/mL) in cell-free ELISA-type assay with dye (A) and non-dye (B) compounds tested. The promiscuous PPI inhibitor erythrosine B (ErB) and the food colorant FD&C yellow no. 6 (sunset yellow, SY) were included as a positive and negative controls, respectively. Data are mean ± SD from two experiments in duplicates and were fitted with standard sigmoid curves for IC50 determination. Estimated IC50s are shown in the legend indicating that while suramin and chloroquine were completely inactive (IC50 > 500 μM), several of our in-house compounds including organic dyes (CgRd, DV1, and others) as well as proprietary DRI-C compounds (e.g., DRI-C23041, DRI-CC91005) showed promising activity, some even at sub-micromolar levels (IC50 < 1 μM).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Importance of the Right Chemical Space
This experiment's success was due to their starting with a different chemical space, which ensured the selected compounds were already effective at low micromolar concentrations. Unlike another recent study that showed 25 possible hits for small molecules that disrupt ACE2-S binding, the current experiment focused on the molecules already known to have a strong binding with proteins.
Interestingly, none of the compounds identified as potential inhibitors in the earlier study showed the common chemical scaffold described above, showing that known drugs or related compounds have a different structure from those known to be SMIs of PPIs.
Advantages of SMIs
A significant advantage of SMIs is their relative non-specificity, which broadens the target for antiviral action. Moreover, they can even neutralize several types of viruses, irrespective of the antigen, through their distinctive action mechanisms. This is very important from the viewpoint of an economic therapeutic approach.
Again, the initial aim of the chemical structures of the drugs developed was to regulate the CD40–CD40L costimulatory interaction at low micromolar potency. Some are selective, others not so much. The finding that they also inhibit ACE2-S PPI shows a non-specific activity, which may still be useful.
The SMIs identified in the current study inhibited ACE2-S complex formation with equal potency in human and mouse tissue.
This indicates that it could be possible to identify SMIs with a broad spectrum of action, including SARS-CoV and MERS-CoV, and other human coronaviruses since in the current study, the target of the SMIs was the Spike protein of both SARS-CoV-2 and SARS-CoV, rather than the ACE2, which is host-specific. This suggests its potential to inhibit infection by both these viruses as well as some other coronaviruses.
Broad Spectrum Antiviral Activity
The SMIs here are mid-range in size but much smaller than those typically identified as PPI SMIs. The increase in size ensures sufficient activity. Interestingly, they tend to not conform to the widely quoted "rule-of-five" criteria for effective pharmaceuticals to ensure high bioavailability and good pharmacokinetics.
One of these is that the molecular weight (MW) should be below 500. Many recently developed drugs similarly show radical departures from these criteria. The researchers say, “Our results provide further proof for the feasibility of SMI for CoV attachment and provide a first map of the chemical space needed to achieve this.”
The researchers describe the potential of their findings: “It could provide a unique opportunity to pursue dual-function molecules that on one hand, have antiviral activity by inhibiting the interaction needed for CoV attachment (e.g., SARS-CoV-2-S–ACE2) and, on the other, possess immunomodulatory activity to rein-in overt inflammation (inhibiting CD40–CD40L) or to unleash T cell cytotoxicity against virus-infected cells (inhibiting PD-1–PD-L1)." The latter is especially significant since T cell balance and restoration are vital to allow adequate viral clearance, prevent immune dysregulation, and avert the cytokine storm so often linked to progressive disease in COVID-19.
Conclusion
The researchers have thus come up with several SMIs with promising inhibitory activity against the S-ACE2 PPI. This action was tested against spike-expressing pseudovirions on cells expressing ACE2 and found to be dose-dependent. Further development may result in an alternative therapy for COVID-19 that can be used orally or by inhalation for treatment and prevention.
Source

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources