Validating the match between the active ingredient content mentioned in the packaging and the actual content in each tablet in a batch is a critical step in tablet manufacture. Highly precise quantitation of the contents may be achieved through various analytical techniques like ion chromatography or titration. The accuracy of these methods, however, depends on the sample preparation methods that precede them. The sample preparation steps prior to the actual analysis vary according to the coating, filling components, and the shape and concentration of the constituents in a pharmaceutical formulation.
Complete homogenization of the tablet using an appropriate solvent is the first step in the preparation of a sample. The need for subsequent steps in sample preparation like additional dilution or pipetting varies according to the analysis technique.
Many of the laboratories execute these steps manually, which is both time intensive, laborious and often ends up with unpredictable results or carryover due to the influence of various circumstances. Use of an automated system goes a long way in overcoming most of these issues by bringing in uniformity in sample handling during sample preparation. A single automation system can take care of not only the quantification of the active ingredient content in a single tablet, but also the product conformity of the entire batch. The advantage of using a fully automated system does not stop with the improvement in accuracy and reliability, but also extends to enhancing the safety in labs and the sample throughput as well.
Instrumentation
One of the most important uricosuric medicines in use is benzbromaron. GC-MS and LC-MS are the advanced methods used for determining benzbromaron, but they are expensive too. Another effective method is titration with sodium hydroxide solution using a simple, completely automated sample preparation process. The fully automated 815 Robotic Soliprep system (Figure 1) has been exclusively designed to suit the sample preparation requirements of HPLC, ICP, titrimetric, ion chromatography applications and others.

Figure 1. 815 Robotic Soliprep system set up
Sample Preparation
The first step in the sample preparation is weighing a definite number of tablets into the sample vessel. The sample data table of the tiamo™ software is then loaded with the analysis data pertaining to the samples that are positioned on the sample rack, such as position, weight and identification. The sample is then transferred to the second workstation for the addition of 60mL methanol just before the pulverizing step. Then, the tablet is pulverized by the Polytron high-frequency homogenizer (Figure 2) for 90 seconds at a speed of 25,000rpm. The corresponding pulverizing time for three tablets is 120 seconds. The homogenized sample is then added to 10mL of water.

Figure 2. The high-frequency homogenizer aggregate with the protruding blade (154mm) is used for sample particles larger than the diameter of the aggregate (12mm). The Polytron homogenizer is made by Kinematica AG, Switzerland.
Titration of benzbromaron is done with the sodium hydroxide solution of 1mol/L with the help of the 809 Titrando and the Solvotrode. Fully automated cleaning is done in the external rinsing station while the titration of benzbromaron is being carried out. The duration of the entire analysis is only 8 minutes.
Benzbromaron Titration
The pKa value of the weak acid benzbromaron is 4.50, which is nearly equal to that of acetic acid with a pKa of 4.75. Resonance stabilization occurs during the titration of benzbromaron (Figure 3), where delocalization of the negative charge at the oxygen atom happens around the ring subsequent to the removal of hydrogen ion. The probability of this occurrence is more when the stability of the ion is higher. Therefore, the determination of benzbromaron can be done effectively by titration using strong bases.
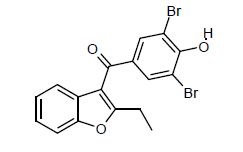
Figure 3. (3,5-dibromo-4-hydroxyphenyl)-2-ethyl-1-benzofuran-3-yl)methanon
Calculation
Calculations are done based on the following equation:
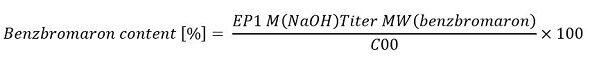
Where EP1 is the titrant consumption till the end point in mL; M is the molarity of NaOH measured in mol/L; Titer is the titrant used, which is a dimensionless value; C00 is the size of the sample in mg; and MW is the molecular weight of benzbromaron in g/mol, which is equal to 421.4g/mol.
Results
Determination with One tablet
Figure 4 shows the plot of the titration results of one tablet and the results are tabulated in Table 1.
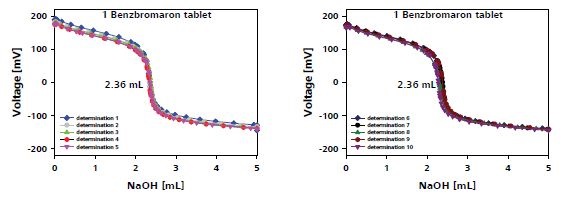
Figure 4. Titration plot for benzbromaron determination in one tablet.
Table 1. Titration results for benzbromaron determination in one tablet
|
Determination
no.
|
Sample weight
[mg]
|
Titration EP1
[mL NaOH]
|
Benzbromaron
|
|
Percentage %
|
Content
[mg/tablet]
|
|
1
|
256.6
|
2.3523
|
38.53
|
98.9
|
|
2
|
256.6
|
2.3653
|
38.74
|
99.4
|
|
3
|
259.1
|
2.3630
|
38.65
|
100.2
|
|
4
|
255.1
|
2.3436
|
38.61
|
98.5
|
|
5
|
256.0
|
2.3763
|
39.01
|
99.9
|
|
6
|
258.4
|
2.3633
|
38.44
|
99.3
|
|
7
|
260.2
|
2.3971
|
38.72
|
100.7
|
|
8
|
260.6
|
2.3252
|
37.50
|
97.7
|
|
9
|
261.0
|
2.3936
|
38.54
|
100.6
|
|
10
|
249.5
|
2.2934
|
38.63
|
96.4
|
|
|
Mean value
|
99.2
|
|
RSD
|
1.36%
|
Determination with Three Tablets
Figure 5 shows the plot of the titration results of three tablets and the results are tabulated in Table 2.
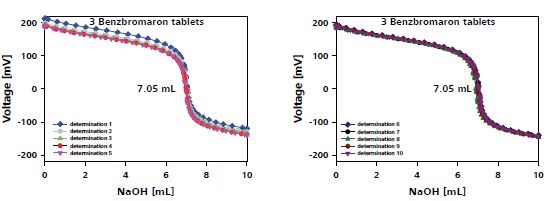
Figure 5. Titration plot for benzbromaron determination in three tablets.
Table 2. Titration results for benzbromaron determination in three tablets
|
Determination
no.
|
Sample weight
[mg]
|
Titration EP1
[mL NaOH]
|
Benzbromaron
|
|
Percentage %
|
Content
[mg/tablet]
|
|
1
|
769.7
|
7.0266
|
38.37
|
98.4
|
|
2
|
771.2
|
6.9992
|
38.14
|
98.1
|
|
3
|
768.2
|
7.1721
|
39.24
|
100.5
|
|
4
|
768.0
|
7.0305
|
38.47
|
98.5
|
|
5
|
771.4
|
7.1075
|
38.72
|
99.6
|
|
6
|
774.3
|
7.0507
|
38.27
|
98.8
|
|
7
|
772.6
|
7.015
|
38.16
|
98.3
|
|
8
|
766.0
|
6.9506
|
38.14
|
97.4
|
|
9
|
773.5
|
7.0211
|
38.15
|
98.4
|
|
10
|
776.6
|
7.0817
|
38.32
|
99.2
|
|
|
|
|
Mean value
|
98.7
|
|
|
|
|
RSD
|
0.88%
|
Conclusion
The content of benzbromaron was found to be 99.2 and 98.7mg per tablet from the ten-fold analysis of one or three tablets, respectively. The RSD value was reduced from 1.36% to 0.88% when three tablets were used instead of one tablet. There was an excellent match between the measured values of benzbromaron and the benzbromaron content provided by the manufacturer, which was 100mg per tablet roughly. In addition to the combination of Titrando and homogenizer discussed in this article, the 815 Robotic Soliprep Sample Processor line offers two other members for end-to-end sample preparation for voltammetry, IC, ICP and HPLC analyses.
Acknowledgements
Produced from materials authored by H. Risse from Metrohm AG, International Headquarters, Herisau, Switzerland.
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.