The release rate and the shelf life of active substances present in tablets are influenced by their water content. Therefore, it is necessary to determine the water content. The dissolution of tablets in Karl Fischer working media, containing alcohol, is difficult.
It requires complex and time-intensive sample preparation methods before the water content can be determined. Consequently, erroneous results may be produced due to the possibility of absorbing moisture more easily from the atmosphere and increased surface area of the tablet subsequent to size reduction.
A method developed by Metrohm allows accurate determination of water content in tablets without the need for complex sample preparation steps. This article discusses the method and the required apparatus.
Advantages of Metrohm’s Method
Complex sample preparation steps are eliminated using a high-frequency homogenizer, which allows the tablets to be reduced into tiny particles directly in the titration vessels that contain the KF solution. The titration vessels are maintained in a fully sealed condition until the analysis begins, thus eliminating the chance of water content alteration caused by atmospheric humidity.
Moreover, the workload is significantly reduced, thanks to the fully automated system (Figure 1). As a result, high sample throughput can be achieved. The sample changers in the system are flexible enough to satisfy the requirements of users, enabling fully automated water content determination in tablets with considerable time and money savings. The easy integration of the high-frequency homogenizer is another advantage of the system.

Figure 1. An overview of the system for automatically determining the water content of tablets
Key Components of the Fully Automated System
The fully automated system for water content determination by volumetric Karl Fischer titration features an 815 Robotic USB Sample Processor XL with two towers, an 841 Titrando, and Kinematica’s high-frequency homogenizer (Polytron).
The Polytron homogenizer has been made compatible with Metrohm instruments, and is installed on the sample changer tower’s robotic titration head (Figure 2). The homogenizer is adjustably placed to the appropriate working height. The sample beakers are emptied by the second sample changer tower upon completion of the analysis. This, in turn, minimizes reagent handling. The Metrodata tiamo™ software control all the components of the fully automated system.

Figure 2. The robotic titration head is equipped with the high-frequency homogenizer, the tubing for the working medium and the indicator electrode. The tablets are treated with the high-frequency homogenizer directly in the sample vessels on the sample changer rack.
Experimental Procedure
The determination procedure is extremely simple, and starts with weighing out a designated amount of tablets directly into the sample vessel based on the expected water content.
The sample vessel is then sealed airtight using a sleeve and aluminum foil and positioned onto the sample changer rack. Next all the relevant sample data is entered into the tiamo™ sample table. It is also possible for automated transmission of the sample weight to tiamo™ by incorporating an appropriate balance.
The start button is pressed for fully automated sample processing. The sample vessel is immediately filled in the working medium before starting the analysis. The high-frequency homogenizer is used to treat the tablets present in the sample vessel for roughly 1-3.5 minutes based on the size and hardness of the tablets. The Karl Fischer reagent is then used for the automated titration of the water discharged by the sample. The reagent volume consumed is recorded in tiamo™.
Since it is possible to reduce the stirring rate of the Polytron homogenizer upon completion of the sample treatment, the homogenizer can also serve as a stirrer while performing the titration, thereby eliminating the need for an additional stirrer.
A rinsing step is performed automatically upon completion of each determination, to prevent the carryover of sample materials by immersing the high-frequency homogenizer into a methanolic solution, where mechanical cleaning takes place by rapid stirring. This is followed by titrating the methanolic cleaning solution to dryness to avoid carryover of any moisture into the next sample.
Aspirating the contents of the sample vessels into a waste container is carried out for subsequent disposal upon completion of the determination series. Water is present in small quantities in the solvents utilized for determination of water content.
Therefore, an automated blank value determination is performed prior to the actual determination. The mean value of several blank determinations is obtained to determine the quantity of KF reagent required for titration of the solvent to dryness. For each sample, the KF reagent consumption is subtracted from the blank value.
Since an approximate titer changing with time is provided by the reagent manufacturer, it is necessary to perform a titer determination for volumetric Karl Fischer titrations.
A Dosino is used to perform this task automatically for measuring out accurately 4mL of a water standard available in the market into an empty sample vessel and quantifying the consumption of the reagent by titration. Determination is performed multiple times and cleaning is not required between the blank and titer determinations.
Results
Three different tablet types of varying water content (1% to 12%) were analyzed in this experiment. Although the tablets have different hardness and size, they could be consistently homogenized, and reproducible results could be produced.
Besides determination of the blank and titer for each type of tablet, determination was successively performed 10 times. The water content measured, for all the samples was in the range anticipated by the manufacturer and showed good agreement with the values determined by utilizing a different system.
A mixture of methanol dry (60%), formamide (39%) and octanol (1%) was used as the working medium. The preliminary results revealed that the solubility of Tablet # 2 was negatively affected by the addition of formamide. Determinations for Tablet #2 were performed using only methanol. The experimental results are summarized in the following tables:
Tablet # 1 (number of tablets per sample is 5)
|
Experiment no.1
|
Blank (mL)
|
Titer (mg/mL)
|
Water content % (w/w)
|
|
|
1.142
|
5.2851
|
0.87
|
|
|
1.114
|
5.3104
|
0.87
|
|
|
1.120
|
5.2851
|
0.88
|
|
|
|
|
0.87
|
|
|
|
|
0.88
|
|
|
|
|
0.87
|
|
|
|
|
0.88
|
|
|
|
|
0.87
|
|
|
|
|
0.88
|
|
|
|
|
0.87
|
|
Mean value
|
1.13
|
5.29
|
0.87
|
|
Standard deviation
|
0.01
|
0.01
|
0.01
|
|
Rel. standard deviation %
|
1.31
|
0.28
|
0.59
|
Tablet # 2 (number of tablets per sample is 1)
|
Experiment no. 2
|
Blank [mL]
|
Titer[mg/mL]
|
Water content % (w/w)
|
|
|
1.402
|
5.2966
|
10.81
|
|
|
1.448
|
5.2576
|
10.79
|
|
|
1.380
|
5.2604
|
10.68
|
|
|
|
|
10.67
|
|
|
|
|
10.68
|
|
|
|
|
10.63
|
|
|
|
|
10.59
|
|
|
|
|
10.85
|
|
|
|
|
10.61
|
|
|
|
|
10.54
|
|
Mean value
|
1.41
|
5.27
|
10.69
|
|
Standard deviation
|
0.03
|
0.02
|
0.10
|
|
Rel. standard deviation %
|
2.46
|
0.41
|
0.95
|
Tablet # 3 (number of tablets per sample is 5)
|
Experiment no. 3
|
Blank [mL]
|
Titer [mg/mL]
|
Water content % (w/w)
|
|
|
0.702
|
5.3898
|
3.80
|
|
|
0.698
|
5.3523
|
3.86
|
|
|
0.710
|
5.3667
|
3.83
|
|
|
|
|
3.84
|
|
|
|
|
3.88
|
|
|
|
|
3.86
|
|
|
|
|
3.88
|
|
|
|
|
3.83
|
|
|
|
|
3.82
|
|
|
|
|
3.79
|
|
Mean value
|
0.70
|
5.37
|
3.84
|
|
Standard deviation
|
0.01
|
0.02
|
0.03
|
|
Rel. standard deviation %
|
0.87
|
0.35
|
0.81
|
Linearity Test
The results were corroborated by checking the linearity of the determination method. Weighing out various quantities of standard substance sodium tartrate dehydrate into sample vessels yielded water contents of roughly 5, 10, 15, 20, 50, 100, 150 and 200mg. The linearity and applicability of the method were corroborated in the range analyzed when the theoretical water contents were plotted against the experimentally determined values (Figure 3).
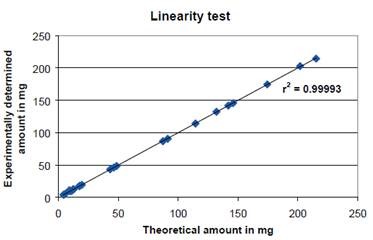
Figure 3. The linearity test showed that the experimentally determined amount of water coincided exactly with the theoretical amount resulting in an outstanding coefficient of determination of R2 = 0.99993
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.