The emergence of peptide therapeutics marks a transformative shift in the types of products being developed and manufactured in the pharmaceutical industry. Peptides, defined by the FDA as biopolymers under 40 amino acids, occupy a unique therapeutic space that offers the specificity and potency of small molecule drugs with the pharmacokinetic benefits of biologics.
Peptide drugs are critical for targeting diseases like diabetes, cancer, cardiovascular disorders, and rare genetic conditions because they mimic endogenous signaling molecules, bind selectively to receptors, and modulate complex biological pathways.
The therapeutic potential of peptides is underscored by the rapid expansion of the TIDES (Therapeutic Peptides and Oligonucleotides) market. The observed market growth for therapeutic peptides (10.77% CAGR between 2024 and 2030) follows the commercial success of GLP-1 agonists. A prominent example of a successfully marketed peptide drug is exenatide (Byetta®), a GLP-1 receptor agonist developed by Amylin Pharmaceuticals and later acquired by AstraZeneca, which generated over USD 500 million in annual revenue during its peak years.
However, this growth is accompanied by the heightened demand for rigorous structural characterization. High-resolution, state-of-the-art techniques are now required to appropriately characterize the increasingly complex structures of peptides that incorporate unnatural amino acids and other modifications. This drive towards enhanced analytics is in response to recently published regulatory guidance documents, which outline higher expectations for identity, purity, strength, and higher-order structure (HOS) assessments, particularly in the context of generic drug development and comparability studies.
In this regulatory landscape, Nuclear Magnetic Resonance (NMR) spectroscopy has emerged as a preferred method due to its ability to provide high-resolution, atom-specific insights into molecular structure and dynamics. NMR offers a non-destructive, solution-phase approach that preserves native conformations and enables multidimensional, high-resolution analysis capable of detecting subtle structural changes, making it indispensable for assessing batch-to-batch consistency, post-translational modifications, and stability.
NMR has historically been leveraged for peptide structure analysis, but improvements in high-field magnets, cryogenically cooled probes, and processing software have dramatically improved sensitivity, resolution, and throughput.
To illustrate the power and versatility of NMR in peptide characterization, this article presents a detailed case study of exenatide, a synthetic GLP-1 receptor agonist used in the treatment of type 2 diabetes. Through a series of 1D and 2D NMR experiments, Bruker demonstrates the ability of NMR to characterize peptide primary and higher-order structure, purity, and stability. These findings not only demonstrate the analytical power of NMR but also underscore its regulatory relevance in the development and lifecycle management of peptide therapeutics.
Materials and methods
A stock solution of exenatide was prepared by dissolving the lyophilized peptide in 10 mM phosphate buffer (pH 7.4) containing 10% D2O (v/v) to a final concentration of 1.0 mg/mL, split into aliquots, and transferred to 3.0 mm and 5.0 mm NMR tubes for analysis. All NMR experiments were conducted on a Bruker Avance Neo 600 MHz spectrometer equipped with a cryogenically cooled 5.0 mm TCI or 3.0 mm MNI CryoProbeTM. The instrument was calibrated using an external DSS standard, and temperature was maintained at 298 K throughout all measurements. One-dimensional (1D) 1H NMR spectra were acquired with water suppression (zgpr) and two-dimensional (2D) experiments, including 1H-13C HSQC, 1H-15N HSQC, 1H-1H TOCSY (mixing time: 60 ms), and 1H-1H NOESY (mixing time: 150 ms), were performed to analyze the peptide’s primary and higher-order structure. All spectra were processed using Bruker TopSpin 4.2 software.
Results
NMR analysis of exenatide
The 1D 1H NMR spectra exhibited well-dispersed amide and aliphatic proton resonances, indicative of a predominantly folded peptide conformation in solution. The 2D experiments confirm the identity of the peptide and the integrity of the sample, which is void of signs of aggregation and degradation. The HSQC spectra revealed sharp, well-resolved cross-peaks for both backbone and side-chain atoms, which can be used as a fingerprint for HOS assessments in stability or comparability studies. The TOCSY and NOESY spectra provided extensive through-bond and through-space correlations, respectively, supporting the presence of secondary structure elements and long-range contacts. Assignments from these experiments can also be used to elucidate the structure of exenatide but is out of scope for this study.
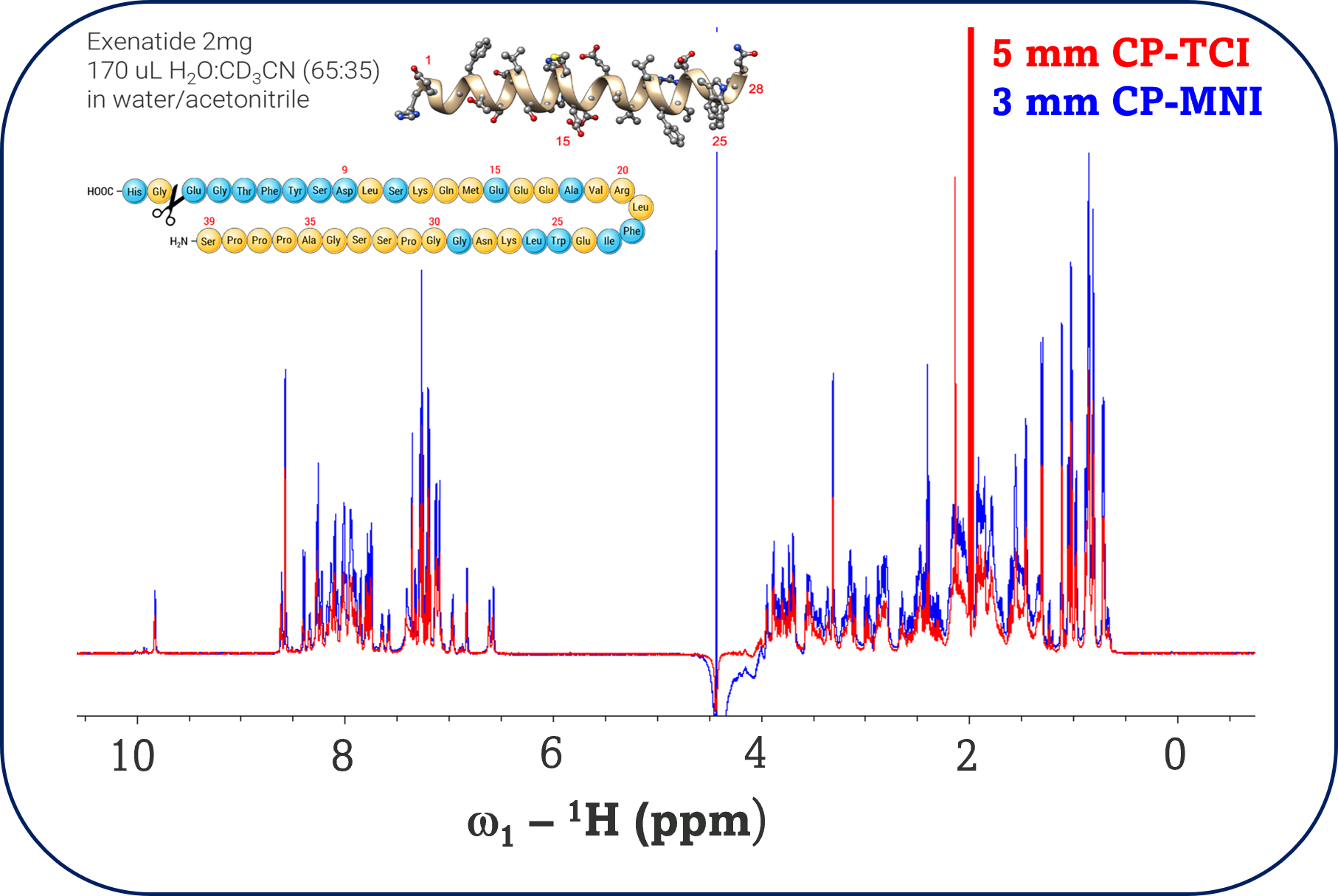
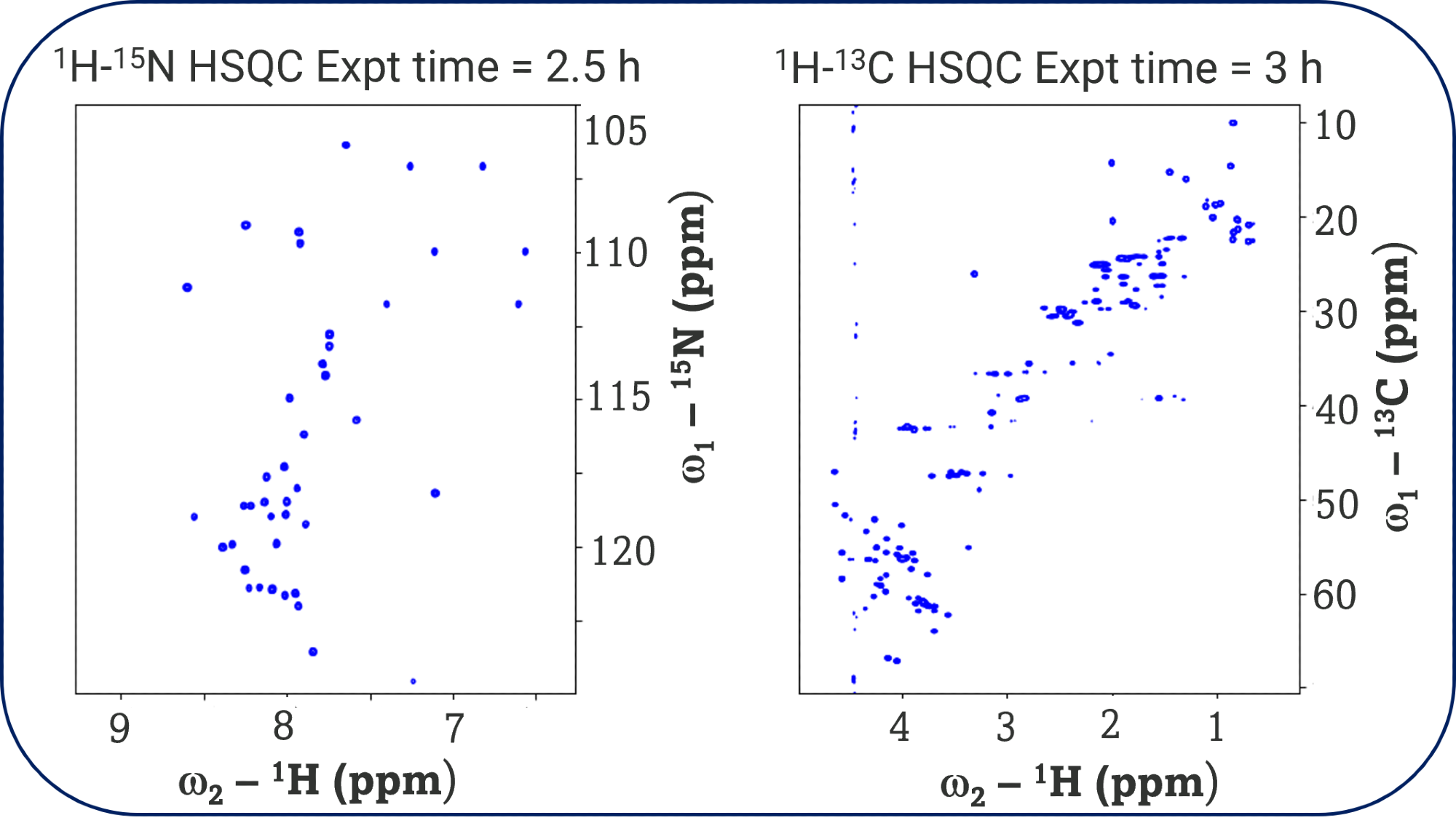
Image Credit: Bruker Biospin Group
Comparison of Probe Technologies: TCI vs. MNI
The Multi-Nuclear Inverse (MNI) CryoProbe™ demonstrated a marked improvement in sensitivity and throughput compared to the conventional TCI probe. Signal-to-noise ratios (SNR) for key amide and aromatic resonances were enhanced by approximately 30-40% with the MNI probe, enabling the acquisition of high-quality spectra from lower sample volumes (3.0 mm tubes) and reduced acquisition times. This sensitivity gain translates directly into improved detection of minor impurities and post-translational modifications, which are critical for regulatory compliance and batch-to-batch comparability.
The ability to acquire multidimensional datasets in a fraction of the time required by conventional probes significantly accelerated the analytical workflow. The enhanced sensitivity of the MNI probe enabled a reduction in acquisition times for the 2D NMR experiments by approximately 40-50% without compromising data quality or resolution, reducing the time to acquire an HSQC spectrum from 4 hours with the TCI probe to 2-2.5 hours. The higher signal-to-noise ratio and improved baseline of the spectra acquired with the MNI probe facilitated more efficient peak picking and assignment during data processing, resulting in reduced post-acquisition data analysis time by an estimated 30-40%.
Regulatory relevance of NMR data for peptide therapeutics
The high-resolution 1D and 2D NMR spectra acquired using both TCI and MNI CryoProbes™ enable unambiguous verification of exenatide’s primary sequence through resonance assignments. The detection of well-dispersed amide and aliphatic proton resonances, along with sharp cross-peaks in HSQC, TOCSY, and NOESY spectra, provides robust evidence for the correct folding and conformational integrity of the peptide in solution. This level of structural detail is essential for demonstrating sameness and comparability to reference listed drugs (RLDs), particularly in the context of generic peptide development.
Conclusion
This study demonstrates the power and versatility of high-resolution NMR spectroscopy for the structural characterization of exenatide, a clinically relevant peptide therapeutic. The comprehensive suite of 1D and 2D NMR experiments enabled a detailed assessment of exenatide’s primary sequence, higher-order structure, and sample integrity. The use of advanced probe technology delivered significant gains in sensitivity, data quality, and workflow efficiency. Future endeavors should focus on advancing automated assignments and structure determination workflows to better analyze emerging modalities, such as peptide-drug conjugates and cyclic peptides.
About Bruker BioSpin Group

Welcome to Bruker BioSpin and the world's most comprehensive range of NMR and EPR spectroscopy and preclinical imaging research tools. The Bruker BioSpin Group of companies develops, manufactures, and supplies technology to research establishments, commercial enterprises, and multi-national corporations across countless industries and fields of expertise.
Bruker BioSpin is continuing to revolutionize the design, manufacture, and distribution of life science, preclinical, process control, and analytical research tools based on magnetic resonance and multimodal imaging technologies. Bruker BioSpin is the global technology and market leader in magnetic resonance technologies (NMR, EPR), offering the largest portfolio of imaging modalities for preclinical and industrial research under a single brand.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.