Tubulin-associated unit (tau) has been widely studied for its role in neurodegeneration since it was first isolated from the porcine brain in 1975.1 Under normal conditions, tau mainly localizes to neuronal axons where it works to promote stability and assembly of microtubules.
Yet tauopathies, a group of neurodegenerative diseases, are characterized by abnormal aggregation and hyperphosphorylation of tau within neuronal cells. Alzheimer’s Disease is the most prevalent of these, it is a condition recently estimated to have a worldwide societal cost of around US $ 1 trillion2. Alzheimer’s Disease currently has no known cure despite significant efforts by researchers.
How can Tau Pathology be Detected?
Alzheimer’s Disease is characterized by an accumulation within the brain of two types of protein aggregate – intraneuronal neurofibrillary tangles consisting chiefly of hyperphosphorylated tau and extraneuronal amyloid fibers made up of the amyloid β peptide.3
For a number of years, the distribution pattern of the neurofibrillary tangles, has formed the basis of a biochemical staging system to differentiate initial, intermediate, and late phase Alzheimer’s Disease4.
Employing Gallyas silver staining of post-mortem sections gathered from different regions of the brain, this method assigns a given Alzheimer’s Disease case one of six defined stages (Braak stages). The silver stain technique has had to evolve to meet the demands of modern laboratories despite widespread use.5
Today, instead of analyzing by eye thick (100 µM) tissue sections embedded in polyethylene glycol (PEG), researchers embed thin (5-15 µM) sections in paraffin and utilize monoclonal antibodies for automated immunodetection.
AT8 was the first of these antibodies to enter mainstream utilization, it has been epitope mapped to the pSer202/pThr205 region of hyperphosphorylated tau.6,7 AT8 has shown proven utility in specifically staining the affected brain regions of patients with Alzheimer’s Disease, though it recognizes the same phosphorylation pattern on the fetal protein.
What is the Role of Phosphorylation at Ser202 and Thr205?
One of a number of post-translational modifications (PTMs) needed for tau aggregation is phosphorylation. Some others include acetylation, ubiquitination, truncation and sumoylation, but to date, phosphorylation is the best characterized PTM in Alzheimer’s Disease.8
The study of tau phosphorylation is extremely challenging, with more than 70 potential phosphorylation sites distributed throughout the entire protein structure, many in clusters. Yet, multiple residues flanking the microtubule binding domains of human tau are particularly implicated in neurodegeneration.9
Of these, the pSer202/pThr205 residues recognized by AT8 have been the focus of a number of studies. It has been recently revealed that in combination with Ser208 phosphorylation and an absence of phosphorylation at Ser262, phosphorylation at these sites generates a protein which readily forms fibers.10
In addition, the levels of pTau at these residues is heightened in Braak stage V/VI, showing that different phosphorylation profiles are associated with Alzheimer’s Disease progression.8
Supporting the Study of Tau Phosphorylation in Alzheimer’s Disease
StressMarq provides a comprehensive portfolio of reagents to support Alzheimer’s Disease research. Included among these, their rabbit monoclonal Tau antibody (pSer202/ pThr205) is a popular alternative to AT8. Benefiting from low background, SMC-601 is validated for immunocytochemistry/immunofluorescence, dot blot, and Western blot.
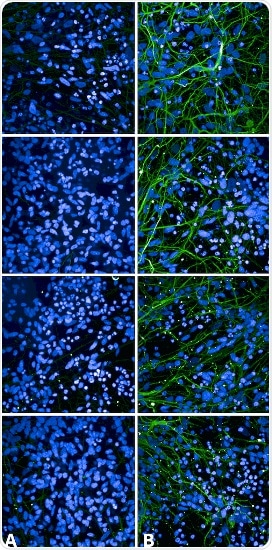
Immunocytochemistry/Immunofluorescence analysis using Rabbit Anti-Tau Monoclonal Antibody, Clone AH36 (SMC-601). Tissue: iPSC-derived cortical excitatory neurons. Species: Human. Primary Antibody: Rabbit Anti-Tau Monoclonal Antibody (SMC-601) at 1:500 for Overnight. Secondary Antibody: Donkey anti-rabbit: Alexa Fluor 488 at 1:1000. Counterstain: DAPI. A) iPSC-derived neurons from non-demented control (NDC). B) iPSC-derived neurons from subject with P301L MAPT mutation. Images acquired using an automated Opera Phoenix system. Each field of view is a max projection from 10 planes of 1 um stacks. Image Credit: StressMarq Biosciences
References and Further Reading
- A protein factor essential for microtubule assembly, Weingarten MD et al, Proc Natl Acad Sci U S A. 1975 May;72(5):1858-62
- https://www.alz.co.uk/research/world-report-2018
- Tau Protein Hyperphosphorylation and Aggregation in Alzheimer’s Disease and Other Tauopathies, and Possible Neuroprotective Strategies, Šimić G et al, Biomolecules. 2016 Jan 6;6(1):6
- Neuropathological stageing of Alzheimer-related changes, Braak H and Braak E, Acta Neuropathol. 1991;82(4):239-59
- Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry, Braak H et al, Acta Neuropathol. 2006 Oct;112(4):389-404
- Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205, Goedert M et al, Neurosci Lett. 1995 Apr 21;189(3):167-9
- A Phosphorylation-Induced Turn Defines the Alzheimer’s Disease AT8 Antibody Epitope on the Tau Protein, Gandhi NS et al, Angew Chem Int Ed Engl. 2015 Jun 1;54(23):6819-23
- Phosphorylation of different tau sites during progression of Alzheimer’s disease, Neddens J et al, Acta Neuropathol Commun. 2018 Jun 29;6(1):52
- Tau Phosphorylation Sites Work in Concert to Promote Neurotoxicity In Vivo, Steinhilb ML et al, Mol Biol Cell. 2007 Dec;18(12):5060-8
- Identification of the Tau phosphorylation pattern that drives its aggregation, Despres C et al, Proc Natl Acad Sci U S A. 2017 Aug 22;114(34):9080-9085
Acknowledgments
Produced from materials originally authored by Patricia Thomson from StressMarq Biosciences Inc.
About StressMarq Biosciences
Established in 2007, StressMarq Biosciences Inc. is a supplier of life science products that operates out of Victoria, Canada with a small, but dedicated group of scientists. Headed by our CEO and President Dr. Ariel Louwrier, StressMarq provides the research community with high-quality reagents backed with rigorous quality control data, expert scientific support, and fast international delivery.
“Discovery through partnership, Excellence through quality”
With over 7,000 products, our growth can be attributed to the continual production of cutting edge research products. Our diverse portfolio of primary antibodies, antibody conjugates, proteins, immunoassay kits and small molecules bridges across the life sciences, including products for cancer research, cardiovascular disease, cell signaling and neuroscience. To aid research worldwide, StressMarq has an extensive network of international distributors that allow us to supply reagents to over 50 countries.
In the years to come, StressMarq will continue to aid life science research by providing “Discovery through partnership, and Excellence through quality”.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.