Introduction
What is GLP-1 and why it matters
Dietary strategies to increase GLP-1
Lifestyle habits that support GLP-1
Supplements and nutraceuticals
Conclusions
References
Further reading
This article explains how specific foods, lifestyle habits, and nutraceuticals can modestly enhance endogenous GLP-1 activity to support appetite regulation and metabolic health. It also clarifies that these natural strategies complement, but do not replace, the effects of GLP-1 receptor agonists.
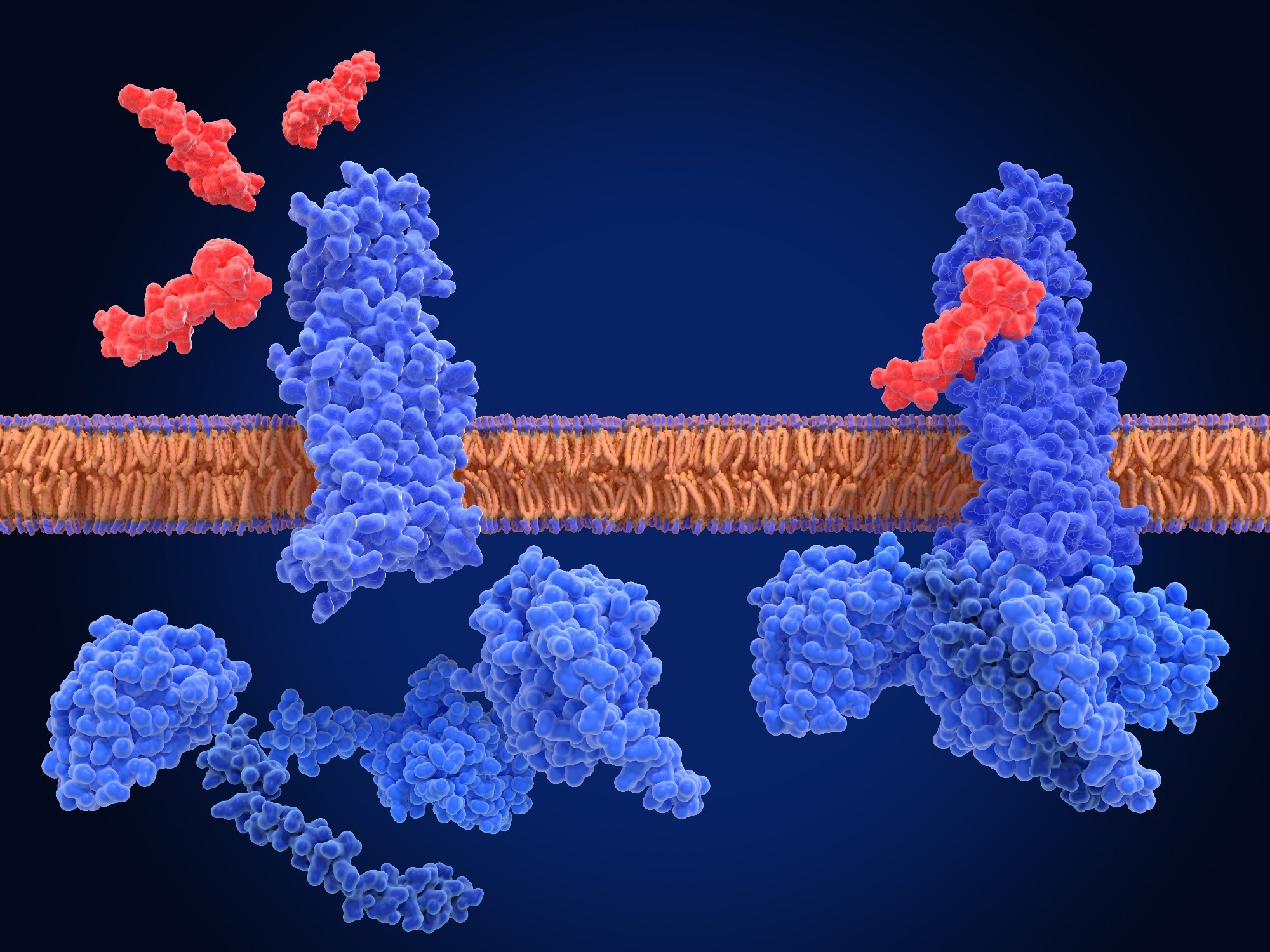 Image Credit: Juan Gaertner / Shutterstock.com
Image Credit: Juan Gaertner / Shutterstock.com
Introduction
Emerging evidence suggests that specific foods, nutrients, and lifestyle habits can stimulate the release of glucagon-like peptide 1 (GLP-1), improve appetite control, and support cardiometabolic balance.1 This article explores evidence-based practical strategies to naturally increase GLP-1 levels to support weight management and metabolic health. Dietary patterns that support GLP-1 biology may also help people tolerate GLP-1 receptor agonist (GLP-1RA) therapy by reducing gastrointestinal side effects, which are a frequent reason for discontinuation in clinical practice.3
What is GLP-1 and why it matters
GLP-1 is an incretin hormone that is primarily secreted by enteroendocrine L-cells in the distal small intestine and colon in response to nutrient ingestion, including carbohydrates, fats, and proteins.1 Smaller amounts of GLP-1 are also produced by neurons in the nucleus tractus solitarius (NTS) of the brainstem, thus highlighting its dual role in both peripheral and central metabolic regulation.1,2
Following a meal, GLP-1 facilitates glucose-dependent insulin secretion from pancreatic β-cells, suppresses glucagon release from α-cells, and slows gastric emptying, all of which moderate glucose excursions following nutrient intake. GLP-1 also acts as a satiety signal by activating receptors on vagal afferent neurons that transmit signals from the gut to the hypothalamic satiety centers, including regions that regulate appetite, energy expenditure, and food reward.1,2
Endogenous GLP-1 is rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4), which results in a short half-life of approximately 1 to 2 minutes.1 Pharmacological GLP-1 receptor agonists (GLP-1RAs) have been developed that mimic the physiological effects of GLP-1 to manage type 2 diabetes and obesity.1,2
How to Thrive in '25: How to Increase GLP1 Naturally with Dr. Melina Jampolis
Dietary strategies to increase GLP-1
Diet plays a pivotal role in modulating endogenous GLP-1 secretion, as certain dietary macronutrients and bioactive compounds can stimulate GLP-1 release from L-cells, thereby regulating appetite and glucose levels.1,5
Protein intake
Dietary proteins are among the most potent natural stimulators of GLP-1 release. Upon digestion, amino acids and peptides activate calcium-sensing and G-protein-coupled receptors on intestinal L-cells.1,5
In particular, whey and casein proteins in dairy products play a key role in promoting satiety and reducing glycemic spikes after meals.1,5 In a study published in Nutrients, a whey protein preload significantly increased GLP-1 and peptide YY (PYY) secretion in young obese women, reduced subjective hunger ratings, and improved postprandial glucose and insulin responses compared with a maltodextrin control, although the acute intervention did not directly assess changes in body mass index (BMI).4
Amino acids like arginine, leucine, and glutamine are also effective in stimulating GLP-1 secretion by activating taste and other receptors on the L-cells that influence intercellular calcium and cyclic adenosine monophosphate (cAMP) signaling pathways.1,5
Fiber and resistant starch
Fermentable fibers and resistant starches undergo microbial fermentation in the colon, resulting in the production of short-chain fatty acids (SCFAs), including acetate, propionate, and butyrate. SCFAs activate free fatty acid receptors FFAR2 and FFAR3 on L-cells, thereby stimulating GLP-1 secretion.1,5
Foods rich in fermentable fiber, such as oats, green bananas, legumes, and cooled potatoes, have been shown to improve fullness and glucose homeostasis. Chronic colonic delivery of propionate, for example, has been associated with reduced body weight and liver fat accumulation in experimental and early human studies.1,5
Healthy fats
Monounsaturated and polyunsaturated fatty acids (MUFAs and PUFAs, respectively) enhance GLP-1 secretion through free fatty acid receptor and G-protein-coupled receptor signaling pathways, including GPR40, GPR119, and FFAR1/3.1,5 Dietary patterns that emphasize healthy fats like olive oil, avocados, nuts, and omega-3 fatty acids effectively maintain GLP-1 activity after meals longer than those heavy in saturated fats, thus supporting better insulin sensitivity and glucose control.1,5
Polyphenol-rich foods
Natural phenolic compounds are increasingly recognized as modulators of incretin pathways. Bioactive plant compounds, such as curcumin and resveratrol, inhibit DPP-4 activity in vitro and in diabetic animal models, which can prolong the active form of GLP-1 and contribute to improved glycemic control and modest weight effects in preclinical settings.6 A small crossover trial in healthy adults also found that a single curcumin-containing drink lowered postprandial glucose excursions, suggesting that polyphenol-rich foods may complement dietary GLP-1 strategies, although GLP-1 itself was not measured.12 These polyphenols provide additional benefits for fat oxidation, insulin sensitivity, and gut microbiota balance, which further support their role in metabolic health.6,12
 Image Credit: New Africa / Shutterstock.com
Image Credit: New Africa / Shutterstock.com
Lifestyle habits that support GLP-1
In addition to diet, several lifestyle factors profoundly influence endogenous GLP-1 secretion and activity. Regular exercise, adequate sleep, and a balanced gut microbiome modulate the incretin pathway, which directly affects metabolic health and weight management.2,5,7,8,13
Exercise
Physical activity enhances GLP-1 secretion and incretin sensitivity through improved L-cell responsiveness and metabolic signaling.2,5 While aerobic exercises burn calories and reduce body fat, resistance exercises involving resistance bands or weights build lean muscle mass, which increases the resting metabolic rate to promote energy utilization, even at rest.5
The Italian Diabetes and Exercise Study (IDES) found that combining aerobic and resistance exercise improved glycated hemoglobin (HbA1c) more effectively than counseling alone, and the Diabetes Prevention Program reports that 150 minutes of weekly activity reduces the risk of type 2 diabetes by 58 percent.5 Mechanistically, exercise-induced interleukin-6 (IL-6) appears to stimulate GLP-1 secretion from intestinal L-cells and pancreatic α-cells in experimental models, which may contribute to improved appetite regulation and insulin secretion during and after exercise.2,5 Moderate or high-intensity exercises for over 30 minutes can transiently increase GLP-1 and reduce ghrelin.5
Intermittent fasting
Intermittent fasting (IF) promotes weight loss by reducing caloric intake and inducing metabolic switching, during which the body transitions from glucose utilization to fat oxidation as its primary source of energy. Time-restricted eating may also improve insulin sensitivity and restore incretin responsiveness over time.5 Randomized data suggest that intermittent energy restriction achieves similar HbA1c reductions to continuous energy restriction, with no clear between-group differences in GLP-1 concentrations, so any specific GLP-1 benefit of IF remains uncertain.5,7
Sleep and circadian rhythm
Adequate sleep is essential for effective weight management, as it regulates appetite-related hormones, supports metabolic function, and promotes energy utilization. Conversely, insufficient sleep can lead to increased appetite, impaired insulin sensitivity, and preferential loss of lean mass over fat during dieting.5,8
Recent data from an observational Japanese study of oral semaglutide users found that sleeping more than six hours per night and improving sleep quality during treatment were independently associated with greater odds of achieving at least 3 percent weight loss, whereas severe stress, late-night dinner after 9 pm, and sleeping within three hours of dinner were linked with poorer weight loss outcomes.8 The authors interpret these findings in the context of prior work showing that sleep restriction alters appetite-regulating hormones such as leptin, ghrelin, and GLP-1, but GLP-1 was not directly measured in this cohort.8
Gut microbiome
Probiotic strains, such as Akkermansia muciniphila and Lactobacillus species, have been linked to improvements in gut barrier function, systemic inflammation, and metabolic markers.5,13 Clinical trials in obesity and non-alcoholic fatty liver disease suggest that multi-strain probiotic formulations can modestly improve anthropometric measures, lipid profiles, and, in some studies, increase GLP-1 concentrations. However, results are heterogeneous and strain-specific.5,13 Meta-analyses indicate that probiotics can produce small but statistically significant reductions in body weight and BMI in adults with overweight or obesity when taken for at least eight weeks, particularly when multiple strains are combined.13 These findings suggest that supporting gut health with probiotics, prebiotics, and fiber may indirectly enhance GLP-1 activity and overall metabolic function.
 Image Credit: Ivanko / Shutterstock.com
Image Credit: Ivanko / Shutterstock.com
Supplements and nutraceuticals
Nutraceuticals that influence incretin pathways may offer complementary benefits to lifestyle modification and pharmacotherapy. Most human trials primarily assess glycemic control, body weight, and lipid parameters rather than GLP-1 directly, so proposed incretin mechanisms often come from preclinical work. Among these products, berberine, chromium, cinnamon, curcumin, and probiotics have the potential to improve glycemic control and, in some cases, to modulate GLP-1 signaling.
Berberine
Berberine is a bioactive alkaloid derived from plants such as Berberis aristata. Upon activation of adenosine monophosphate (AMP)-activated protein kinase (AMPK), berberine enhances insulin sensitivity, reduces hepatic glucose output, and has been shown in animal models to increase GLP-1 secretion, although this mechanism has not yet been confirmed in large human trials.5,6
In a 12-week randomized trial, supplementation with 1,200 mg per day of berberine and 600 mg per day of cinnamon significantly reduced fasting blood glucose, HbA1c, and low-density lipoprotein (LDL) levels in patients with type 2 diabetes, without producing significant changes in body weight or BMI.9 These clinical findings are consistent with preclinical data that berberine activates AMPK and favorably alters lipid metabolism, but the trial did not directly measure GLP-1 or gut microbiota composition.5,6,9
Chromium and cinnamon
Chromium is an essential trace mineral that enables insulin signaling and glucose uptake by enhancing insulin receptor activity. Randomized studies have shown that chromium supplementation, often in combination with other compounds, can modestly improve markers of glycemic control. In a randomized, placebo-controlled trial in overweight or obese pre-diabetic adults, a supplement containing cinnamon, chromium, and carnosine significantly reduced fasting plasma glucose and increased fat-free mass compared with placebo. However, HbA1c did not differ between groups.10
Acute cinnamon intake may also influence GLP-1 and postprandial responses. In a crossover study in healthy subjects, a 3 g cinnamon dose added to a rice pudding test meal slowed gastric emptying and reduced postprandial glucose, insulin, and GLP-1 concentrations compared with control, while self-reported satiety was modestly increased.11 These short term effects suggest that cinnamon can alter gut hormone dynamics, though the long term impact on weight and GLP-1 biology is not established.10,11
Curcumin
Preclinical and in vitro data suggest that curcumin can inhibit DPP-4 activity and enhance glycemic control in diabetic animal models, potentially prolonging the active life of GLP-1, although this has not been confirmed in human mechanistic studies.6 In a randomized, controlled, crossover study in healthy subjects, consuming 180 mg of curcumin before a carbohydrate-rich meal significantly reduced the incremental area under the curve for plasma glucose over 120 minutes by approximately 36 percent compared with control, without major adverse events.12 Nevertheless, curcumin’s effects on circulating GLP-1 have not yet been directly quantified in human trials, so its role as a GLP-1 targeted strategy remains speculative.6,12
Conclusions
Balanced meals that combine fiber, protein, and healthy fats can naturally increase GLP-1 activity, promoting satiety, maintaining optimal energy levels, and improving glucose control.1,4,5 Conversely, diets rich in refined carbohydrates and processed foods may suppress this beneficial response.1,5,13
Thus, a diet that frequently incorporates nutrient-dense foods, such as vegetables, legumes, nuts, eggs, and whole grains, supports metabolic balance. Structured exercise, adequate sleep, and targeted use of probiotics and selected nutraceuticals provide additional, evidence-informed levers to complement pharmacological GLP-1RAs or to support metabolic health in individuals who are not using these medications.2,5,8-10,12,13 As research advances, these insights highlight the potential of nutrition science to inform future GLP-1-targeted therapies and sustainable strategies for metabolic health.
References
- Bodnaruc, A.M., Prudhomme, D., Blanchet, R. et al. (2016). Nutritional modulation of endogenous glucagon-like peptide-1 secretion: a review. Nutrition and Metabolism 13, 92. DOI:10.1186/s12986-016-0153-3, https://nutritionandmetabolism.biomedcentral.com/articles/10.1186/s12986-016-0153-3.
- Kanoski, S. E., Hayes, M. R., and Skibicka, K. P. (2016). GLP-1 and weight loss: Unraveling the diverse neural circuitry. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. DOI:10.1152/ajpregu.00520.2015, https://journals.physiology.org/doi/full/10.1152/ajpregu.00520.2015
- Gentinetta, S., Sottotetti, F., Manuelli, M., and Cena, H. (2024). Dietary Recommendations for the Management of Gastrointestinal Symptoms in Patients Treated with GLP-1 Receptor Agonist. Diabetes, Metabolic Syndrome and Obesity 17; 4817. DOI:10.2147/DMSO.S494919, https://www.dovepress.com/dietary-recommendations-for-the-management-of-gastrointestinal-symptom-peer-reviewed-fulltext-article-DMSO.
- Rigamonti, A. E., Leoncini, R., Casnici, C., et al. (2019). Whey Proteins Reduce Appetite, Stimulate Anorexigenic Gastrointestinal Peptides and Improve Glucometabolic Homeostasis in Young Obese Women. Nutrients 11(2); 247. DOI:10.3390/nu11020247, https://www.mdpi.com/2072-6643/11/2/247.
- Fujiwara, Y., Eguchi, S., Murayama, H., et al. (2019). Relationship between diet, exercise, and pharmacotherapy to enhance the GLP-1 levels in type 2 diabetes. Endocrinology, Diabetes and Metabolism 2(3); e00068. DOI:10.1002/edm2.68, https://onlinelibrary.wiley.com/doi/full/10.1002/edm2.68
- Huang, K., Lin, R., Chang, H., et al. (2019). Natural phenolic compounds potentiate hypoglycemia via inhibition of Dipeptidyl peptidase IV. Scientific Reports 9; 15585. DOI:10.1038/s41598-019-52088-7, https://www.nature.com/articles/s41598-019-52088-7.
- Coutinho, S. R., Halset, E. H., Gasbakk, S., et al. (2018). Compensatory mechanisms activated with intermittent energy restriction: a randomized control trial. Clinical Nutrition 37(3); 815-823. DOI:10.1016/j.clnu.2017.04.002, https://linkinghub.elsevier.com/retrieve/pii/S0261-5614(17)30125-5.
- Takakura, K., Koda, N., Kinoshita, Y., et al. (2025). Impact of Sleeping Habits on the Weight Loss Effect of Oral Glucagon-Like Peptide-1 Receptor Agonists Among Obese Patients: An Observational Study in Japan. Cureus 17(7); e87823. DOI:10.7759/cureus.87823, https://www.cureus.com/articles/385333.
- Mansour, A., Sajjadi-Jazi, S. M., Gerami, H., et al. (2025). The efficacy and safety of berberine in combination with cinnamon supplementation in patients with type 2 diabetes: a randomized clinical trial. European Journal of Nutrition 64(102). DOI:10.1007/s00394-025-03618-9, https://link.springer.com/article/10.1007/s00394-025-03618-9.
- Liu, Y., Cotillard, A., Vatier, C., et al. (2015). A Dietary Supplement Containing Cinnamon, Chromium, and Carnosine Decreases Fasting Plasma Glucose and Increases Lean Mass in Overweight or Obese Pre-Diabetic Subjects: A Randomized, Placebo-Controlled Trial. PLoS One 10(9). DOI:10.1371/journal.pone.0138646, https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0138646.
- Hlebowicz, J., Hlebowicz, A., Lindstedt, S., et al. (2009). Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. The American Journal of Clinical Nutrition 89(3); 815-821. DOI:10.3945/ajcn.2008.26807, https://www.sciencedirect.com/science/article/pii/S0002916523237533.
- Thota, R. N., Dias, C. B., Abbott, K. A., et al. (2018). Curcumin alleviates postprandial glycaemic response in healthy subjects: A crossover, randomized controlled study. Scientific Reports 8(1); 1-8. DOI:10.1038/s41598-018-32032-x, https://www.nature.com/articles/s41598-018-32032-x.
- Wicinski, M., Gebalski, J., Golebiewski, J., and Malinowski, B. (2020). Probiotics for the Treatment of Overweight and Obesity in Humans - A Review of Clinical Trials. Microorganisms 8(8); 1148. DOI:10.3390/microorganisms8081148, https://www.mdpi.com/2076-2607/8/8/1148
Further reading
Further Reading
Last Updated: Nov 13, 2025