CRISPR: A primer
CRISPR and human disease
Technical challenges and considerations
Ethical and regulatory implications
The future of CRISPR therapy
References
Further reading
CRISPR: A primer
CRISPR, or Clustered Regularly Interspaced Short Palindromic Repeats, is an advanced technology developed in 2012 that can be used to edit genes. It can be used to find specific DNA sequences inside cells in order to modify DNA. This innovative technology has also been adapted for applications such as turning a gene ‘on’ or ‘off’ without changing its sequence.1
While there were previous gene editing techniques before the establishment of CRISPR, these were enormously time-consuming, taking years to gain traction and costing hundreds of thousands of dollars. However, the development of CRISPR has revolutionized this process, providing a cheaper and simpler technique for gene editing, which is already being widely used in scientific research.1
The key component of the CRISPR system includes the Cas9 protein, which is found in bacteria where it defends against viruses. The Cas9 protein can be easily programmed to identify and bind to any targeted DNA sequence by providing a piece of RNA as a guide for its search. The standard Cas9 protein then cuts the DNA at the target site, and when the cut is repaired, mutations are introduced, which typically deactivate the gene.1
Image Credit: MicroOne/Shutterstock.com
This application of CRISPR is used the most and is known as genome or gene editing; however, the results are not as precise as they seem. While CRISPR can be used to make more precise alterations, such as replacing dysfunctional genes, which is true genome editing, this process is more difficult.1
CRISPR and human disease
The potential of CRISPR application for treating genetic disorders may be monumental, with this application enabling treatment for diseases such as sickle cell anemia, cystic fibrosis, and even cancer. This therapeutic application would be revolutionary for the healthcare of the global population, with genetic disorders and congenital abnormalities being present in 2-5% of births worldwide and being responsible for up to 50% of all early childhood mortality.2
Sickle cell anemia consists of an inheritable autosomal recessive disorder with a mutation that replaces an amino acid on chromosome 11, with glutamic acid being replaced by valine. This single amino acid substitution causes the formation of hemoglobin S, which is the disease-causing form of hemoglobin. Subsequently, it leads to deformed blood cells that adopt a crescent shape that looks like a sickle under a microscope.2
The rigid, distorted red blood cells cause all the symptoms of sickle cell disease by obstructing blood vessels, inhibiting blood flow, and causing severe pain in areas that are not receiving adequate circulation. This obstruction also results in clogs in the heart, lungs, liver, and eyes.2
While there is no defined treatment for those living with sickle cell, there are two FDA-approved medications that reduce the severity of the disorder. However, with the emergence of CRISPR, two major therapeutic applications can work to treat sickle cell, including either repairing the genes for hemoglobin S or replacing hemoglobin S with hemoglobin F.2
CRISPR-Cas9 can also be used to fix the dysfunctional CFTR gene that causes cystic fibrosis. With CRISPR-mediated gene repair, its functionality can be restored, preventing respiratory failure caused by the disease.2
In 2018, the first CRISPR Phase I clinical trial in the United States opened, with the aim being to use CRISPR/Cas9 to edit autologous T cells for cancer immunotherapy against many different cancers with relapsed tumors, but without a further requirement of curative treatment options.3
The use of CRISPR in infectious diseases such as HIV has also been explored. The principle behind this is to reduce the HIV-1 infection as well as remove proviruses by targeting cellular cofactors, which was used for the first time in 2013 for treating HIV-1/AIDS.2
The potential for CRISPR technology to target other infections caused by viruses is also significant, as it is usually very difficult to eliminate viruses after they have found a place to replicate within a host cell. As a result, CRISPR-based approaches can be used to target viral sequences to prevent the viruses from being able to go through transcription or replication. The potential for CRISPR gene therapy can be used globally and may even revolutionize healthcare in developing countries.2
Technical challenges and considerations
While CRISPR technology may enable advancement in medicine and biomedical research, there are also some limitations, including its off-target effects. The high frequency of off-target effects is a major concern for the implementation of CRISPR for gene therapy.3
However, there are current attempts that aim to overcome this limitation, including engineered Cas9 variants that demonstrate decreased off-target effects and the optimization of guide designs. This includes an approach that utilizes Cas9 nickase, which is a variant that induces single-stranded breaks in combination with a single guide RNA pair targeting both strands of DNA at the target site to produce a double-stranded break.3
The delivery modality for gene therapy is also a challenge, as this has a significant impact on safety as well as its therapeutic efficacy. Traditional gene therapy uses viruses as a delivery vehicle; however, this consists of risks such as immunotoxicity and insertional oncogenesis.3
Adeno-associated virus (AAV) vectors are used as the delivery carrier for CRISPR gene therapy, with the CRISPR system being packaged as plasmid DNA encoding its components such as Cas9 and guide RNA or delivered as mRNA of Cas9 and the guide RNA.3
Nucleic acids of CRISPR can be packaged in AAV vectors for delivery or delivered into target cells through electroporation or microinjection, with the latter approach eliminating virus-associated risks.3 However, the conditions of the latter methods may also be challenging as every CRISPR therapeutic need requires a significant amount of genome-editing enzyme into cells, which may mean delivering multiple macromolecules at the same time.3,4
Additionally, there are also concerns about innate immune responses to the guide RNA within the CRISPR system, which has been addressed through phosphatase treatment.4
However, another challenge consists of humans potentially having pre-exposure to the same antigens as Cas9 and the delivery vectors, such as adenoviral vectors, that are needed to carry the effectors for targeted therapy. This exposure may trigger the body’s humoral immunity, which is mediated by antibodies or cellular immunity involving cytotoxic T cells. This may cause serious adverse reactions, death of Cas-expressing cells, and ineffective CRISPR treatment.4
Ethical and regulatory implications
Somatic editing for CRISPR therapy has been enabled after significant consideration. However, human germline editing for therapeutic use is still very controversial. This is because somatic editing risks can be contained within the person. However, with embryonic editing, any unforeseen and permanent side effects can pass down into offspring through generations, without autonomy in the decision-making process of those of the next generation.3
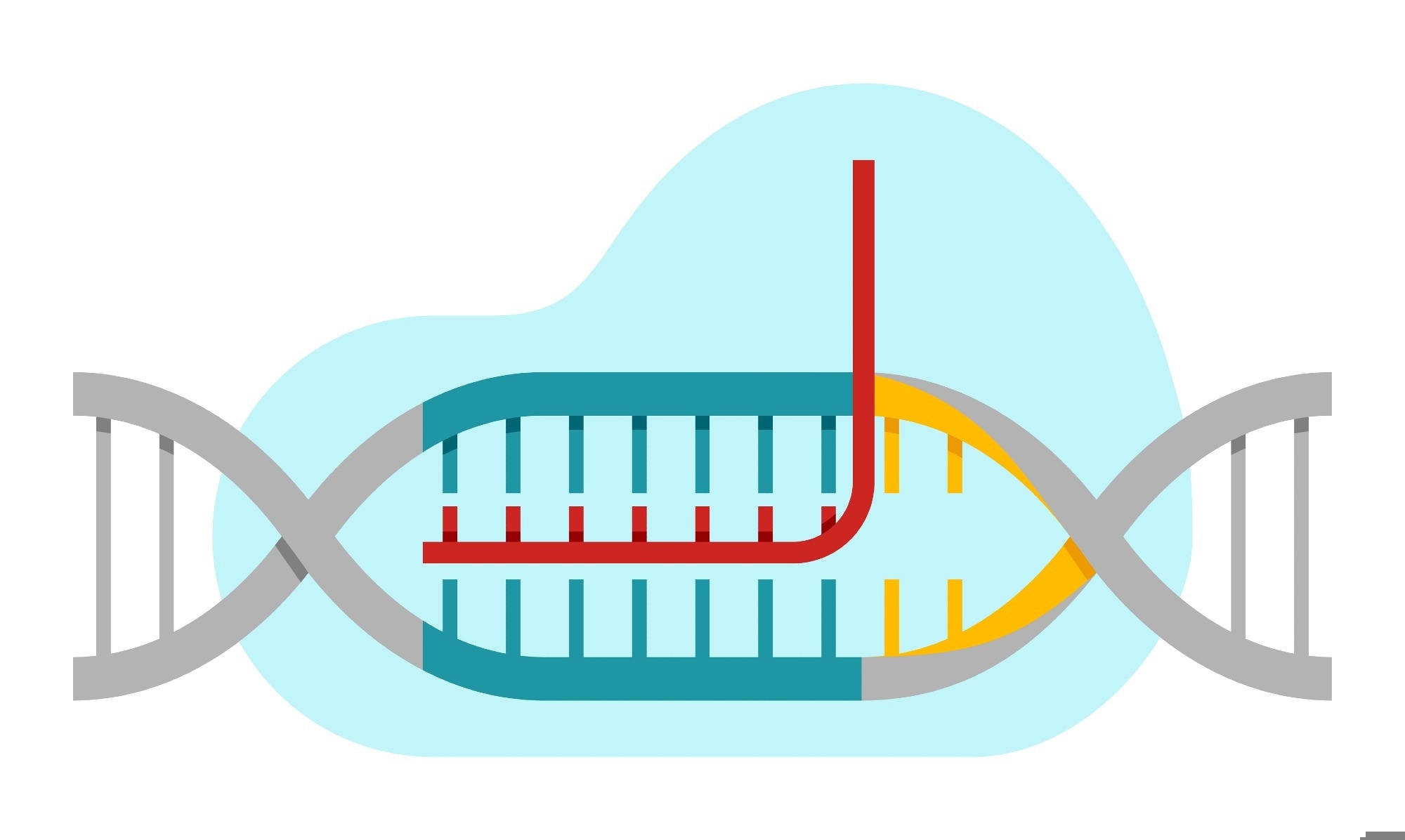
Image Credit: Dmitry Kovalchuk/Shutterstock.com
CRISPR-Cas germline editing therapies (CGETs) development can be categorized into three phases, including preclinical research, clinical trials, and post-approval distribution. Before starting research on any therapy, scientists are required to obtain funding and approval from institutional ethics boards. CGETs must be assessed from the start by funding bodies and institutional regulators to check for safety and patient benefit before research can begin.5
Additionally, preliminary safety standards for research require verification of the specificity of CRISPR-induced modifications in a model cell line and ensure new DNA that is introduced into genomes passes through generations at a normal rate. This is because one of the largest risks of CGETs is unintended side effects from new alleles that are only found generations after the initial gene editing process.5
The future of CRISPR therapy
Most CRISPR-based cancer-related trials include therapeutic studies that are in Phase I or II clinical trials, with 54% of studies being for hematologic malignancies and 46% being for solid tumors.6
Adoptive cell transfer (ACT) of tumor-reactive T lymphocytes, including chimeric antigen receptor (CAR)-T cells, T cell receptor-engineered T (TCR-T) cells and tumor-infiltrating lymphocytes (TILs), have been reported to be one of the most promising immunotherapeutic methods for cancer, and has been researched in 86% of CRISPR-based clinical trials, with the aim to be engineered by Cas9 ribonucleoprotein (RNP) to increase their therapeutic ability.6
The potential for CRISPR technology is significant in medicine for many diseases, which enhances the therapeutic possibility for a global population that may not have had an effective treatment available thus far. From cancer to sickle cell disorders, CRISPR technology may revolutionize current healthcare therapeutics.2,6
References
- Le Page M. What is CRISPR? New Scientist. Published 2020. https://www.newscientist.com/definition/what-is-crispr/
- Luthra R, Kaur S, Bhandari K. Applications of CRISPR as a potential therapeutic. Life Sciences. 2021;284(119908):119908. doi:https://doi.org/10.1016/j.lfs.2021.119908
- Uddin F, Rudin CM, Sen T. CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future. Frontiers in Oncology. 2020;10(1387). doi:https://doi.org/10.3389/fonc.2020.01387
- Liu W, Li L, Jiang J, Wu M, Lin P. Applications and challenges of CRISPR-Cas gene-editing to disease treatment in clinics. Precision Clinical Medicine. 2021;4(3):179-191. doi:https://doi.org/10.1093/pcmedi/pbab014
- Evitt NH, Mascharak S, Altman RB. Human Germline CRISPR-Cas Modification: Toward a Regulatory Framework. The American Journal of Bioethics. 2015;15(12):25-29. doi:https://doi.org/10.1080/15265161.2015.1104160
- Zhang S, Wang Y, Mao D, et al. Current trends of clinical trials involving CRISPR/Cas systems. Frontiers in Medicine. 2023;10. doi:https://doi.org/10.3389/fmed.2023.1292452
Further Reading
Last Updated: Feb 24, 2025