Nov 20 2014
FDA clears new device for use along with a standard colonoscope to improve visibility in areas behind folds in colon wall
Avantis Medical Systems, Inc., a technology leader in developing novel digital imaging devices, today announced that its Third Eye® Panoramic™ device for colonoscopy has received 510(k) clearance from the U.S. Food & Drug Administration (FDA).
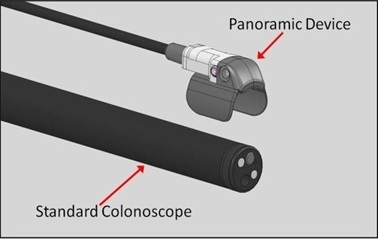
Third Eye Panoramic device attaches to the tip of a standard colonoscope
The Third Eye Panoramic device utilizes technology that was developed for the company’s earlier product, the Third Eye® Retroscope®. Like its predecessor, the Panoramic device is designed to help physicians see behind folds in the wall of the colon that can hide pre-cancerous polyps called adenomas, but with improved ease-of-use and reduced expense.
While the Retroscope device had one additional camera that was aimed backward, the new Panoramic device instead has two video cameras that are directed laterally from its left and right sides. The video monitor displays the lateral images on both sides of the colonoscope’s forward image, resulting in an ultra-wide-angle view of more than 300 degrees. This “panoramic” view reveals areas behind folds and flexures (sharp turns) in the colon.
The Third Eye Panoramic device is attached to the tip of the colonoscope at the beginning of the procedure, and it can be used during both the insertion and withdrawal phases. Because it clips onto the outside of the colonoscope, it leaves the channel completely free, in contrast to the earlier device that occupied the channel of the colonoscope and had to be removed when the physician inserted instruments to remove polyps.
“We spent thousands of hours observing endoscopists as they performed colonoscopies using the Third Eye Retroscope, and we listened to their concerns regarding the ease of use,” said Jack Higgins, MD, Chief Medical Officer for Avantis Medical Systems. “They liked the fact that additional cameras allowed them to see more of the colon, and they asked us to find a way to do that more easily and inexpensively, and without occupying the channels of their colonoscopes.”
Dr. Sang Kim and Dr. Moshe Rubin recently performed a feasibility study of the Third Eye Panoramic device at New York Hospital Queens Weill Cornell Medical College. They found that the Panoramic device worked well in conjunction with a standard colonoscope and provided enhanced, wide-angle imaging of the colon without adverse events. The results of the second phase of that study demonstrated an adenoma detection rate of 48% and were presented at the American College of Gastroenterology Annual Scientific Meeting (ACG 2014) in Philadelphia on October 21.
“Improving the quality of colonoscopy with enhanced imaging has been shown in recent studies to increase our ability to detect precancerous polyps. The elegant approach offered by Avantis Medical in developing the Third Eye Panoramic cap offers significant advantages. Gastroenterologists can continue to utilize their existing high definition standard colonoscopes without new investments in capital equipment,” said Dr. Rubin. “Additionally, the non-obtrusive video cap does not change the dynamics of scope insertion or maneuverability, thus providing an enhanced nearly 360-degree view without sacrificing scope familiarity, usability or efficiency.”
The Third Eye Panoramic device is designed to be used along with any standard adult or pediatric size colonoscope, so it avoids any substantial capital expense and it allows physicians to continue using the high-quality technologies in which their facilities have already invested, generally including colonoscopes with high-resolution or even high-definition video cameras.
“Now that the Third Eye Panoramic device has been cleared by the FDA for marketing, it will allow other leaders in the field of gastroenterology to provide us with feedback as we develop a resposable version of the device,” said Doug Gielow, Avantis’ Vice President for Sales & Marketing. “At the same time we can finalize our selection of a partner with broad domestic and international commercial distribution that is beyond the reach of a development company.”