Mar 28 2017
Drugs containing gold have been used for centuries to treat conditions like rheumatoid arthritis. In addition, they might be effective against cancer and HIV. One mechanism by which they work could occur because gold ions force the zinc ions out of zinc fingers—looped, nucleic acid binding protein domains. American researchers have characterized such “gold fingers” using ion mobility mass spectrometry. As reported in the journal Angewandte Chemie, they identified the exact gold binding sites.
“The zinc ions in zinc fingers bind to four sulfur or nitrogen atoms of the protein’s cysteine and histidine residues,” explains Nicholas P. Farrell of Virginia Commonwealth University (Richmond, USA). “Gold ions bind to just two amino acid fragments and change the conformation of the protein. The “gold fingers” are no longer able to bind to nucleic acids, which may be therapeutically useful.”
Although there are a variety of potential binding sites for metal ions, each metalloprotein usually prefers a single conformation. It was previously not possible to determine where the specific binding sites were in a mixture of conformers. Farrell and his team have now closely examined two gold fingers. According to Farrell, “replacing the zinc in zinc finger 3 of Sp1 transcription factor leads to only a single gold finger species.” The researchers identified this as having a linear Cys-Au-His bond. In the case of the HIV nucleocapsid protein, which plays a critical role in the replication of the virus, “putting gold in the zinc finger 2 of the protein (NCp7-F2), leads to three different gold finger species with linear Cys-Au-Cys motifs, one of which is clearly predominant.”
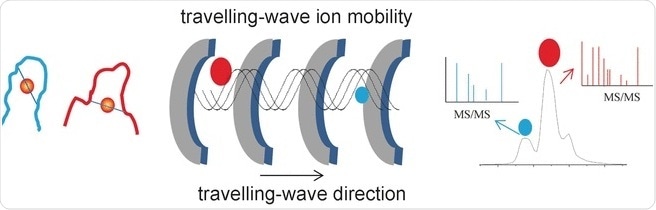
The researchers’ success stems from their use of a special analytical method called travelling-wave ion mobility mass spectrometry (TWIM-MS). In this technique, the molecules to be analyzed are ionized and the ions accelerated by an electric field in a gas. Collisions with the gas molecules cause the ions to be slowed. Large, voluminous molecules are slowed more than small, compact ones because they collide more frequently. This makes it possible to differentiate and separate isomers as well, because although they have the same mass, their different geometries result in different mobility. Once separated according to their mobility, the individual ions can now be fragmented through collision induced dissociation (CID) and the fragments measured again by mass spectrometry. This makes it possible to characterize shorter peptides that remain bound to gold.
“In this way, we were able to identify the specific binding sites and modes for the gold-modified zinc fingers NCp7-F2 and Sp1-F3,” says Farrell. “Ion mobility mass spectrometry thus provides important information about the changes in geometry caused by the exchange of zinc in the zinc finger proteins, as well as the selectivity and reactivity of such reactions. This could be of benefit in the search for new metal-based antiviral and antitumor drugs.”