Even as the COVID-19 pandemic continues to cost thousands of lives and causes millions of cases worldwide, studies are going on to establish safe and effective remedies for the disease.
There have been seven coronaviruses that caused illness in humans, including the past MERS and SARS outbreaks. However, there is no vaccine against any coronavirus strain at present, and even natural immunity has not been proved to be sustained. Earlier research during the SARS epidemic suggests that reinfection can occur.
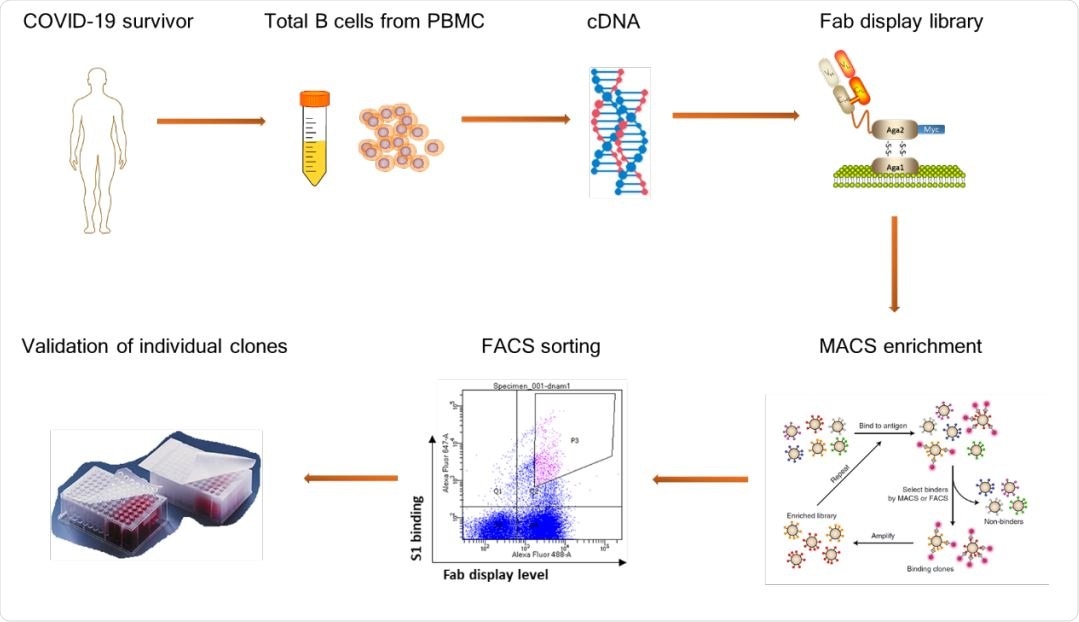
A schematic diagram showing the workflow of neutralizing antibody discovery

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Convalescent Plasma
Since vaccine trials are not known to proceed rapidly due to the intrinsic nature of the development process, scientists are turning towards other therapeutic options. One is the administration of convalescent plasma, which has been proved to be safe and has effectively reduced the rate of clinical complications like respiratory symptoms in patients with severe COVID-19.
Nonetheless, this is unlikely to be a workable solution on a large scale since it depends on the availability of enough donors with a high concentration of anti-SARS-CoV-2 antibodies, and facilities to process serum to neutralize any other viral pathogens before transfusion. However, many pharmaceutical firms and academic researchers have explored the possibility of isolating neutralizing antibodies from recovered patients since the start of the current year.
Overall, these monovalent antibodies have a weak affinity for the viral spike protein, which makes it needful to screen numerous antibodies to find one which has a powerful neutralizing ability. Another approach is to use computational approaches to achieve affinity maturation in silico so as to obtain a higher level of binding affinity.
Other Options: Antibody Cocktails and rACE2
Some teams have resorted to using a mixture of neutralizing antibodies to ensure adequate neutralization, but this increases the cost and difficulty of manufacture. Another technique often used is to block the host cell receptor for the virus, namely, angiotensin-converting enzyme (ACE2).
The use of recombinant ACE2 has proved successful in reducing the load of viral particles and viral growth rates in cultured cells and in organoids, by mimicking the natural receptor. However, the bivalent homodimer of ACE2 called APN01 has too short a half-life and unfamiliar mode of interaction with the spike (S) protein of the virus to provide effective blockade.
Novel Fusion Protein 89C8-ACE2
To work around this, researchers have used a fusion protein, composed of ACE2 fused with an Fc domain, to form a recombinant bivalent ACE2 molecule with a longer half-life and increase its affinity for the viral S1 protein, which in turn makes it more potent at inhibiting viral entry. The current study describes the novel biparatopic molecule designed from the fusion of the neutralizing antibody 89C8 with recombinant ACE2 (89C8-ACE2). This fusion molecule binds to the N-terminal domain (NTD) of the S1 subunit of the S protein.
The advantages of this molecule are its higher binding affinity and neutralizing ability shown on both pseudotyping and virus infectivity assays. The design is also important for its potential to block other coronaviruses as well since it does not bind to the receptor-binding domain (RBD) of S1 but to the NTD. The RBD is an area that can escape binding via mutations. By avoiding this, the researchers offer a more universal design for a therapeutic drug that could be extended to other infectious diseases as well.
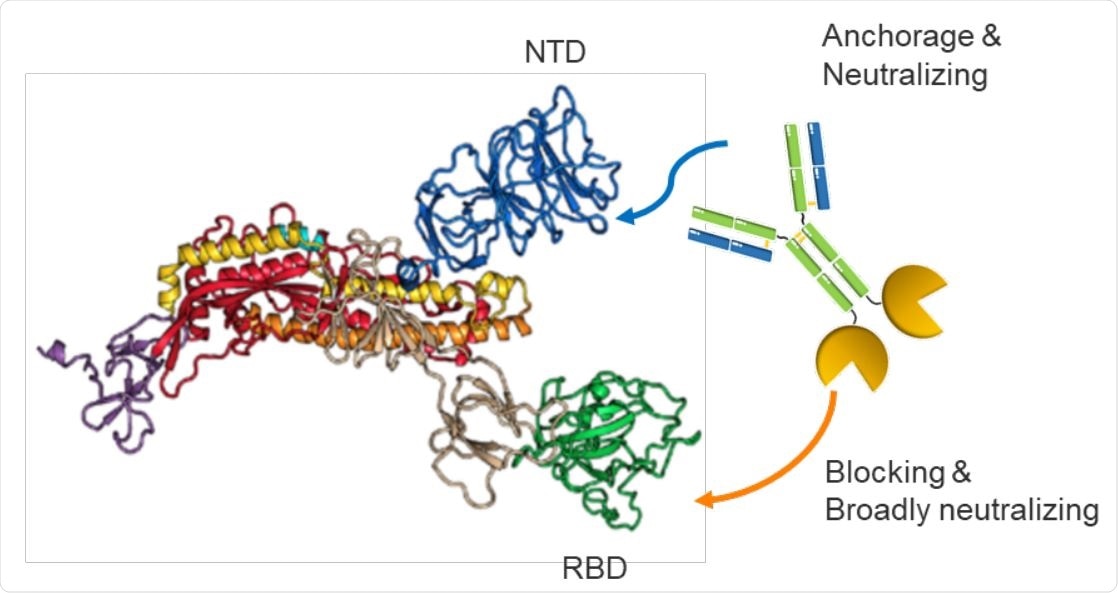
Schematic illustration of the biparatopic 89C8-ACE2 antibody-fusion design
Selecting the Antibody
The first step was to isolate the specific antibodies against this virus, which was carried out on blood specimens from ten donors. Three of them had a strong antibody response to the recombinant S1 protein, which was used to identify the presence of specific memory B cells against the virus. These cells were used to construct three libraries of antibody sequences.
These libraries were used for yeast display screening, with the recombinant S1 protein fused to the Fabs of specific anti-S1 antibody molecules. These antibody molecules were now selected by coated magnetic beads and sorted to yield, finally, 115 unique Fab sequences in pairs. Among these, 50 were selected to produce a monoclonal antibody (mAbs) for further testing.
These 50 mAbs were allowed to bind to the cultured cells, which expressed the whole S1 protein sequence and characterized.
The researchers then focused on producing a recombinant anti-S1 ACE2 fusion protein that had biparatopic properties, capable of binding two epitopes simultaneously. This would, therefore, be better at binding S1. The aim was to produce antibodies capable of blocking S1-ACE2 interaction. Here, the antibody 89C8 showed better and faster binding characteristics and Fab quality.
Building the Biparatopic Construct
They engineered a biparatopic molecule wherein 2 ACE2 molecules are fused with a linker to the C-terminal domain (CTD) of the 89C8 heavy chain. Just for comparison, molecules comprising ACE2 fused to the NTD of the light chain or the heavy chain of the antibody. Only the CTD-bound molecules displayed strong binding.
When 89C8 was tested by itself, the binding was strong and sustained. With the ACE2-Fc molecule, the binding is described as “fast on-fast off.” But with the biparatopic construct 89C8-ACE2, binding was both sustained and multivalent, with stronger binding affinity as a result compared to the monovalent antibody, and possibly longer protection.
The ACE2-Fc is a natural inhibitor of the S1/ACE2 binding but with weak monovalent binding affinity. It can block the binding of soluble SARS-CoV-2 S1 protein with ACE2, but the construct was found to have a hundred times more potency. 89C8 failed to block this interaction at all.
Potent Neutralizing Capacity
When the construct was tested for neutralization of SARS-CoV-2 infection, it was found to completely neutralize the pseudotyped virus, similar to ACE-Fc, and 89C8 achieved 90% neutralization. However, the neutralization titer was markedly lower compared to the other two, showing its potency. It also proved its neutralization capacity in cell cultures infected with the native virus, reducing plaques by 50% and 90% at 1.7nM and 6.2 nM, respectively, compared to 3.7 nM and 33.3 nM, respectively, for ACE2-Fc. It also outperformed the neutralizing potential of a nanobody fusion construct, BHH72-Fc, that has broad neutralization ability against betaCoV, binding the S1-RBD.
Advantages and Applications of the Construct
The use of the NTD is a powerful blocking tool against SARS-CoV and SARS-CoV-2, being a common target due to the high homology. The use of recombinant ACE2 as a decoy is suggested by the fact that soluble ACE2-Ig, or recombinant soluble human ACE2, can inhibit infection with this virus. Moreover, ACE2 also protects the lung against injury during COVID-19 infection.
The use of double copies of ACE2 with the neutralizing antibody exploits two mechanisms, namely, direct blocking of ACE2-S1 interaction, and locking it against conformational change, with extremely avid binding. The construct has powerful multivalent binding which is sustained for up to 2 hours, with exceptional avidity due to its binding to both the NTD of the S1 as well as its RBD simultaneously.
The researchers comment, “Antibody combinations targeting distinct epitopes may act synergistically resulting in the reduction of the dosage required for therapeutic potency as well as the risk of immune escape through mutational changes.”
With picomolar inhibitory concentrations, and superior neutralizing activity compared to the native antibody, recombinant ACE2 or even VHH72-Fc, the 89C8-ACE2 molecule is a promising lead for therapeutic development for COVID-19 as well as other infectious diseases.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources