The pace and magnitude of the COVID-19 pandemic have led to an unprecedented search for a safe and effective vaccine for SARS-CoV-2 infection or drug for COVID-19 treatment. Many are searching among already approved drugs to repurpose them for the current need. This includes hydroxychloroquine, dexamethasone, and remdesivir.
Autophagy Inhibitors
One promising direction of research is inhibition of autophagy, a mechanism diverted by many viruses, including coronaviruses, to allow viral replication. Coronaviruses have been found to use vesicles with bilayered membranes to facilitate viral propagation. Some think that they induce autophagy.
The current study developed an inhibitor of autophagy, GNS561, a small molecule that causes dysregulation of the lysosome, leading to the inhibition of autophagy at a late stage. This drug from Genoscience Pharma, Marseille, France, is being developed for cancer therapy, but it is also being evaluated for COVID-19.
The researchers aimed to assess GNS561’s in vitro antiviral activity. They found that it was the most potent antiviral in vitro, when tested against SARS-CoV-2, compared to remdesivir and chloroquine. Its EC50 was 0.006 μM, with a large margin of safety before cytotoxicity sets in. This is 16 and 200 times lower than that of chloroquine and remdesivir, respectively.
Autophagy inhibition by GNS561
The researchers found that treatment with GNS561 induces accumulation of and enlargement of autophagic vacuoles in the cytoplasm, with multilamellar characteristics. They found that SARS-CoV-2 was found in lysosomes positive for LAMP2, similar to GNS561. After infection with SARS-CoV-2, LAMP2 particles were observed to enlarge, due to alterations in the expression of intracellular LC3-II or ‘spots’, in a dose-dependent manner. This indicates the impact of the treatment on the process of autophagy.
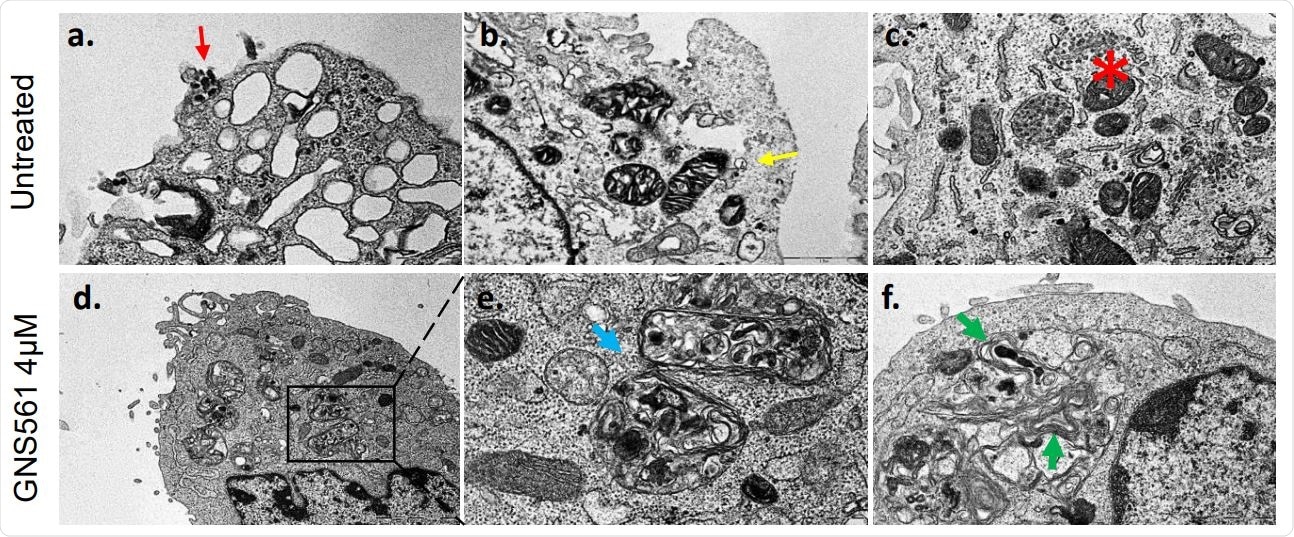
GNS561 leads to autophagy blockage After 2 hours of treatment with different doses, Vero E6 cells were infected with SARS-CoV-2 IHU-MI6 strain. (A) Electron microscopy pictures illustrating infected cells without (upper panel) or with GNS5561 treatment (lower panel). The presence of the virus is indicated using a red arrow, endocytic vesicles in the cytoplasm with clathrin-coated vesicles with yellow arrows, autophagy vacuole with blue arrows and multilamellar bodies with green arrows. Vacuoles filled with nascent particles are illustrated using a red asterisk.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
They also found indications that cell infection by SARS-CoV-2 is mediated by autophagic flux. Thus, GNS561 treatment caused a dose-dependent increase in LC-III expression, which shows the drug modulated autophagy in a specific manner. The researchers postulate that the drug alters autophagosome synthesis, and overall, it inhibits viral proliferation.
Remdesivir-GNS561 a Powerful Synergistic Inhibitor Combination
The researchers found that when Vero cells were treated with either drug alone, at different concentrations, or in combination, there was a strongly synergistic inhibitory effect on viral replication at all tested concentrations of GNS561 and remdesivir concentrations between 0.1 and 0.5 μM.
Implications
This compound appears to have among the most potent antiviral inhibitory activity observed among all tested compounds so far. By inhibiting autophagy, it prevents the hijacking of the autophagy mechanism by SARS-CoV-2 in the early stages of the infection. Further work is required to understand how this drug works against this virus.
The use of drug combinations is highly recommended to prevent the emergence of resistance and escape mutations. This demonstration of strong synergy supports further evaluation of its clinical activity in patients with COVID-19 infection in the early phase, before cytokine dysregulation sets in. Its efficacy at low levels, coupled with its high cytotoxicity concentration, with a good safety profile in Phase II presents many advantages that promote its further study as a possible addition to the currently available meager armamentarium against COVID-19. Moreover, its possibly broad spectrum of action may indicate that it could be developed against future global pathogens as well.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources