The severity of COVID-19 among the residents of long-term care facilities (LTCFs) has been reported to be disproportionately high. Specifically, individuals residing in skilled nursing facilities (SNFs) are found to be the most severely affected, both in terms of morbidity and mortality, and are, thereby, listed for early COVID-19 vaccination. In comparison to the general population, SNF residents are more vulnerable, with a weak immune system.
LTCFs and SNFs were not included in the clinical trials of COVID-19 vaccines. This resulted in a limitation, i.e., minimal data to determine the vaccine effectiveness (VE) for this vulnerable group. Additionally, in the case of the Pfizer-BioNTech vaccine, the immunogenicity was found to be lower in the age group between 65–85 years. However, for the younger population, the vaccine was found to be more efficient.
The Center for Disease Control (CDC) - Pharmacy Partnership for Long-Term Care Program has facilitated the on-site vaccination for the residents and staff of SNFs. This program involves end-to-end COVID-19 vaccination management. Several organizations are LTCFs, federal partners, including the Centers for Medicare and Medicaid Services (CMS), and the American Health Care Association (AHCA) are working closely on this program.
On December 18, 2020, SNF residents and staff members in Connecticut had started receiving the Pfizer-BioNTech COVID-19 vaccine. The Connecticut Department of Public Health (CT DPH) had identified two SNFs (facility A and facility B) undergoing COVID-19 outbreaks in late January 2021. While investigating the situation, CT DPH, in partnership with CDC, conducted a retrospective cohort investigation and obtained electronic chart data of these facilities. In this study, a total of 463 residents were enrolled, containing 142 (31%) from facility A and 321 (69%) from facility B. The electronic data included details such as demographics, vaccination dates, presence of pre-existing medical conditions associated with potential increased risk for severe COVID-19 illness, hospitalization for COVID-19, and death.
The study, reported in the CDC's Morbidity and Mortality Weekly Report (MMWR), was conducted from the date of each SNF’s first vaccination clinic, i.e., December 29, 2020, for facility A and December 21, 2020, for facility B, and ended on February 9, 2021, and February 12, 2021, respectively. All SNF residents, regardless of symptoms, were tested for COVID-19 using the polymerase chain reaction (PCR) test. In facility A, residents were tested once a week, and residents in facility B were tested twice a week. The staff members of both the SNF facilities were also tested regularly. Additionally, in both facilities, additional antigen testing was immediately conducted for any resident or staff member who showed COVID-19 symptoms or those who had been exposed to a COVID-19 patient.
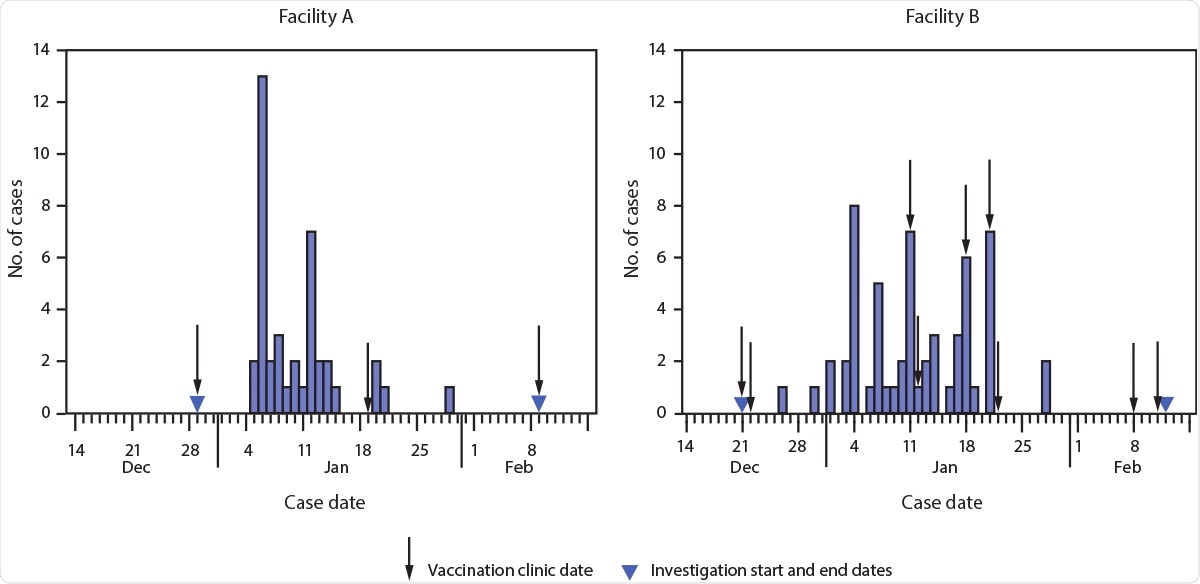
New SARS-CoV-2 cases among residents of two skilled nursing facilities, by case date — Connecticut, December 21, 2020–February 12, 2021
The current study revealed that the estimated effectiveness of partial vaccination in preventing COVID-19 disease was 63% (95% confidence interval [CI] = 33%–79%) among residents and staff of these SNFs. Serological studies have shown that a single dose of the COVID-19 vaccine would develop a stronger response for individuals with pre-existing immunity. Additionally, towards the end of the study period, after the SNF residents were fully vaccinated. i.e., seven days following the second dose, researchers observed an insufficient number of new cases. The remaining unvaccinated group served as a comparative reference for estimation of full 2-dose VE.
This study revealed the efficacy of a single dose of the Pfizer-BioNTech COVID-19 vaccine in older adults and other age groups in non-congregate settings. Scientists have suggested that to optimize vaccine impact among this population, high coverage with the complete 2-dose series should be recommended for SNF residents and staff members.
This study has several limitations, some of which are as follows. Racial minority groups were underrepresented and ethnic data were not available. Estimating two-dose VE was not possible as the number of unvaccinated cases and person-time observations after the second dose of the vaccine were insufficient. Small sample sizes posed limitations for analyses of secondary endpoints (e.g., the vaccine's effectiveness against symptomatic illness and hospitalization).
The current investigation from this retrospective cohort analysis showed that partial vaccination with the Pfizer-BioNTech COVID-19 vaccine could significantly reduce the risk for SARS-CoV-2 infection among SNF residents. Similar studies that included the older age group in Israel have reported more efficient protection against COVID-19 infection after completing the second vaccine dose. However, this study's most significant importance lies in the data validation and accurate accumulation of vaccination data using electronic medical records and active ascertainment of SARS-CoV-2 infection through frequent testing of the SNFs residents and staff. In the future, researchers recommend using a more extensive study sample for analysis.