Finding effective and safe vaccines is crucial in the fight against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes the coronavirus disease 2019 (COVID-19).
Researchers at the National Center for Nanoscience and Technology of China, the Chinese Academy of Sciences (CAS), introduced a new vaccine, which compromises the spike protein’s receptor-binding domain (RBD) and the manganese nanoadjuvant (MnARK), inducing humoral and cellular responses.
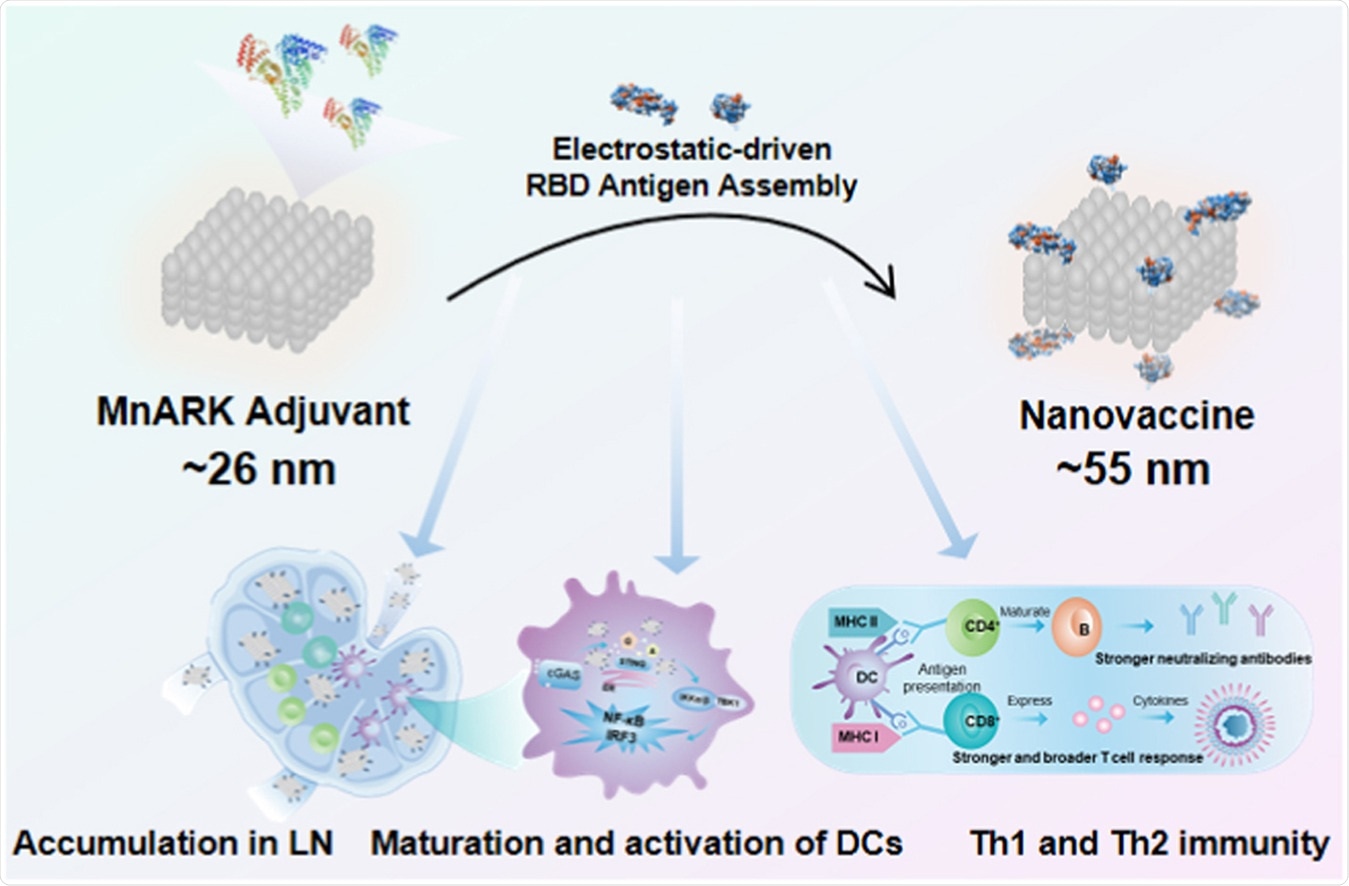
Graphical Abstract. Image Credit: Nanotoday / ScienceDirect
The study, recently published in the journal Nanotoday, highlights the potential of a MnARK-based nanovaccine to prevent SARS-CoV-2 infection. The vaccine is a programmable platform that can enhance the co-delivery efficiency of RBD antigen and a nanoadjuvant to lymph nodes.
Study background
More than 125.85 million cases of COVID-19 have been reported worldwide since the virus was first detected in Wuhan, China, in December 2019. Of these, 2.76 million people have lost their lives. One effective way to prevent infection is through vaccination.
Multiple novel coronavirus vaccines have shown promising results, with many being used in vaccine campaigns in many parts of the world.
Yet, the limited production capacity would negatively impact herd immunity and slow down pandemic control measures. One way to boost vaccine production and use is by using a suitable adjuvant to the subunit vaccine, which can improve weak RBD immunogenicity, reduce the number of vaccinations and antigen dosage, and trigger potent neutralizing antibodies to combat the pandemic.
Currently, Alum adjuvant is the only approved and safe one used. It helps the antigen to produce a more robust immune response than a free antigen. The lack of cellular immune response limits Alum-formulated vaccines.
However, the manganese (Mn) nanoparticle adjuvant may be another suitable candidate. Exhibiting the potential to stimulate innate immune responses, it has been used in developing cancer vaccines.
To date, the design and structure of vaccine systems that provide satisfactory antigen delivery and immune cell activation are significant concerns for protein subunit-based vaccines. The unique size of functional nanomaterials aid in the delivery of vaccine components to vital immune cells or lymphoid tissues, improving the immune response to combat infection.
Meanwhile, LNs are restrictive in size, making it hard to deliver the vaccine to immune cells. Hence, designing a simple approach to delivery the antigen and adjuvant and activating the adaptive and innate immune responses are essential for novel coronavirus subunit vaccines.
The study
The researchers constructed a nanovaccine through albumin to concurrently deliver antigen and adjuvant to lymph nodes. The team noted that an antigen-adjuvant-formulated nanovaccine, at about 10 to 100 nm, would accumulate in LNs after injection.
The constructed nanovaccine contains the RBD antigen of the S1 protein and the manganese nanoadjuvant (MnARK), a negatively charged cubic manganese oxide nanoparticle known to active the cGAS-STING pathway and transport RBD antigens to the lymph nodes.
Compared with the traditional Alum-adsorbed RBD vaccine, the nanovaccine markedly increased RBD-specific immunoglobulin G (IgG) and immunoglobulin M (IgM) responses by 10-fold and 5-fold, respectively. It also enhanced the neutralization of the SARS-CoV-2 based on both pseudovirus and live virus, by 270-fold and 8-fold, respectively.
Further, the nanovaccine induced wider and more robust T-cell responses than the conventional Alu-RBD vaccine, stimulating the cGAS-STING pathway and inducing a high-quality immune reaction.
Importantly, a potent neutralizing antibody response induced by MnARK nanovaccine strongly supports its promising potential for realizing a satisfactory protective immunity against novel coronavirus,” the researchers wrote in the paper.
The low cost of the nanovaccine makes it possible for clinical investigation in future studies. The team hopes the nanovaccine can be used to combat the spreading pandemic, as it has many advantages over other types of vaccines.
The combination of an adjuvant with the RBD protein in a MnARK nanovaccine may be an ideal strategy for the development of vaccines to combat novel coronavirus or other coronaviruses,” the researchers added.
Source:
Journal reference: