Researchers in the United States have identified several clinically approved compounds that could be repurposed for the treatment and prevention of coronavirus disease 2019 (COVID-19).
By screening a commercial library of drugs that have already been approved by international regulatory agencies, the team identified more than 50 compounds that demonstrated some efficacy in blocking the initial stage of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the causative agent of COVID-19.
The compounds were able to disrupt the binding of a surface viral protein called Spike to its host cell receptor angiotensin-converting enzyme 2 (ACE2).
The researchers – from Seattle Children’s Research Institute in Washington and St. Louis University School of Medicine in Missouri – say that although the compounds are currently unlikely to be viable for treating or preventing COVID-19, they could serve as starting points for the future design of effective therapeutics.
“These results highlight an effective screening approach to identify compounds capable of disrupting the Spike-ACE2 interaction as well as identifying several potential inhibitors that could serve as templates for future drug discovery efforts,” says Stephen Smith and colleagues.
A pre-print version of the research paper is available on the BioRxiv* server, while the article undergoes peer review.
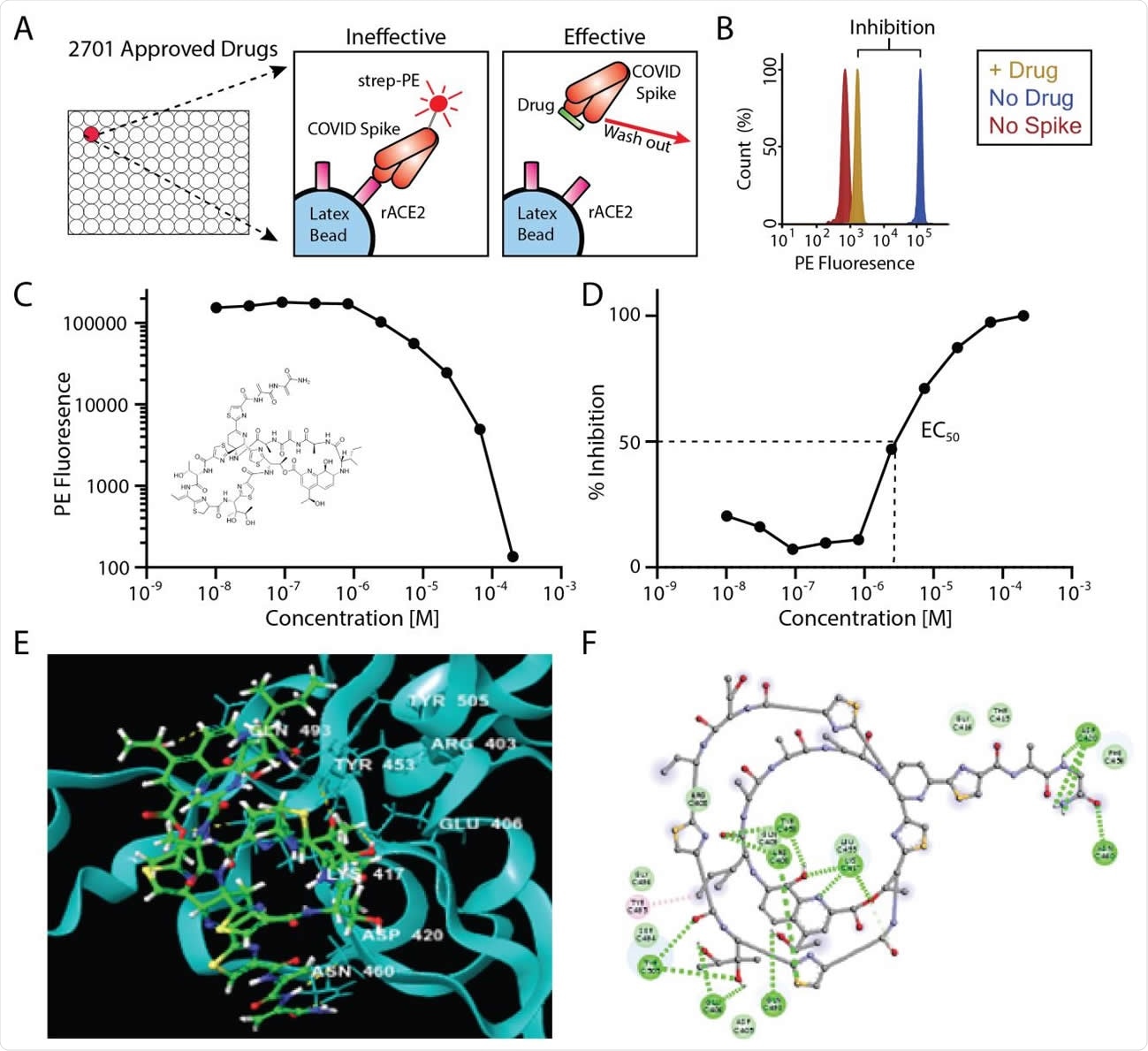
Inhibition of Spike-ACE2 binding by repurposed drugs. A) In vitro assay design showing inhibition of ACE2-Spike binding by “effective” drugs. B) Example histogram from primary screen showing >90% inhibition of ACE2-Spike binding following drug addition. C, D) EC50 data for the top candidate, Thiostrepton (structure inset) expressed as PE fluorescence (C) or percent inhibition (D). E) Three-dimensional and F) two-dimensional computational rendering of Thiostrepton binding to the SARS-CoV-2 spike protein.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Repurposed drugs could offer a rapid route to novel treatments and prophylactics
The COVID-19 pandemic continues to pose an unprecedented threat to global public health, having now caused more than 135 million infections and more than 2.93 million deaths since the outbreak began in Wuhan, China, in late December 2019.
While the success of rapidly developed vaccines shows promise in controlling the pandemic, repurposed drugs that block binding of the SARS-CoV-2 spike protein to ACE2 could offer a rapid route to novel COVID-19 treatments and prophylactics.
“Repurposing already-approved small molecule drugs could allow for rapid deployment of low-cost and widely available therapeutics, but candidates studied in robust clinical trials have thus far failed,” writes Smith and the team.
What did the researchers do?
The researchers screened 2,701 compounds from the “FDA-approved drug screening library” for their ability to inhibit the binding of recombinant, trimeric SARS-CoV-2 spike protein to recombinant human ACE2.
Using a previously validated assay, the team quantified the level of the Spike-ACE2 interaction in the presence of each drug and calculated the percentage inhibition of the binding interaction.
Smith and colleagues identified 114 compounds that inhibited Spike-ACE2 binding by 90% or more.
Serial dilutions of the compounds were performed to measure the EC50s (concentration of a drug that gives 50% of its maximum response) of the binding inhibition.
This led to the elimination of 58 compounds that either showed no inhibition upon re-screening or only demonstrated inhibition at the highest concentration tested.
Of the 56 drugs remaining, the lowest EC50s were observed for thiostrepton (a topical antibiotic used to treat animals) and oxytocin (a hormone used to induce childbirth).
The next best candidates were nilotinib (a treatment for chronic myelogenous leukemia) and the cancer drug hydroxycamptothecine.
Modeling the docking of the most effective inhibitors
The team computationally modeled the docking of the 12 most effective inhibitors to both Spike and ACE2.
The compounds with the lowest EC50s also gave the lowest docking scores (predictive of higher binding affinity), thereby cross-validating the team’s results.
The two top hits – thiostrepton and oxytocin – bound more favorably to Spike than to ACE2 and interacted with several key residues that mediate the Spike-ACE2 interaction.
Nilotinib and hydroxycamptothecine bound slightly more favorably to ACE2, although they also exhibited a moderate affinity for the Spike. Interestingly, both of these compounds interacted with the critical Spike-binding residue Arg393 and also interacted with the Spike receptor binding interface.
The remaining compounds generally gave higher docking scores, consistent with their lower binding affinity for Spike and ACE2.
A starting point for drug discovery efforts
“Overall, this study identified 56 approved drugs that show some efficacy in blocking the interaction between the COVID spike protein and its receptor, ACE2,” say the researchers. “Many of the identified drugs are already approved for clinical use in humans.”
However, several problems exist in terms of clinical translation, says the team.
Firstly, many of the drugs are chemotherapy agents with toxic side effects that would not be tolerated among COVID-19 patients.
Secondly, several drugs have peak plasma concentrations that are orders of magnitude lower than would be required to inhibit Spike-ACE2 binding.
“The relatively weak binding kinetics of the drugs identified here, as well as their known toxicities, bioactivities and/or high clearance rates, suggest that many would currently be unlikely to be viable for treating acute disease or for prophylactic use,” writes Smith and colleagues.
“However, they could serve as starting points for future medicinal chemistry optimization efforts to rationally design derivatives that are both less toxic and bind to the COVID spike with higher affinity,” they conclude.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources