Researchers in the United States have identified short peptide sequences in the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that are immunogenic in a mouse model and may provide protection against viral variants.
Researchers at the Massachusetts Institute of Technology (MIT) set out to address the increasing concerns that current vaccines designed to protect against SARS-CoV-2 infection and subsequent coronavirus disease 2019 (COVID-19) may be less effective against the viral variants that have emerged.
These “variants of concern” contain mutations in the viral spike protein – the main structure that mediates the initial stage of the infection process by binding to host cells.
“A concern is that existing SARS-CoV-2 spike subunit vaccines produce neutralizing antibodies to three-dimensional spike epitopes that are subject to change during viral drift,” they write.
Now, David Gifford and colleagues have provided preliminary evidence to support the hypothesis that adaptive T cell-based immunity may provide a path for the development of a pan-COVID-19 vaccine that is resilient to viral drift.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.

Assembled vaccine construct containing a secretion signal sequence (red), peptides (bold) joined by non-immunogenic glycine/serine linkers, and an MHC class I trafficking signal (blue).
Emerging variants have motivated the search for new vaccines
Since the COVID-19 outbreak first began in Wuhan, China, in late December 2019, several SARS-CoV-2 variants have emerged that have been shown to increase transmissibility and to escape the immune response generated by vaccination.
Studies of spike variants tested as pseudoviruses have indicated increased resilience to the neutralizing antibodies produced by spike-based vaccines or natural infection with the original viral strain.
“The ultimate clinical implications of these findings are at present unclear, but they have motivated a search for vaccination methods that are resilient to new strains of SARS-CoV-2,” writes Gifford and team.
What did the researchers do?
The researchers hypothesized that an alternative to vaccine-induced antibody-based neutralization of SARS-CoV-2 would be vaccination that generates a robust cellular immune response to protect against symptomatic infection.
“T-cell-based adaptive immunity can be based on short peptide sequences selected from the viral proteome that are less subject to drift, and can utilize multiple such epitopes to provide redundancy in the event of drift,” writes Gifford and colleagues.
Now, the researchers have proposed T cell-based vaccines that have previously been predicted to produce both CD8+ and CD4+ T cell responses.
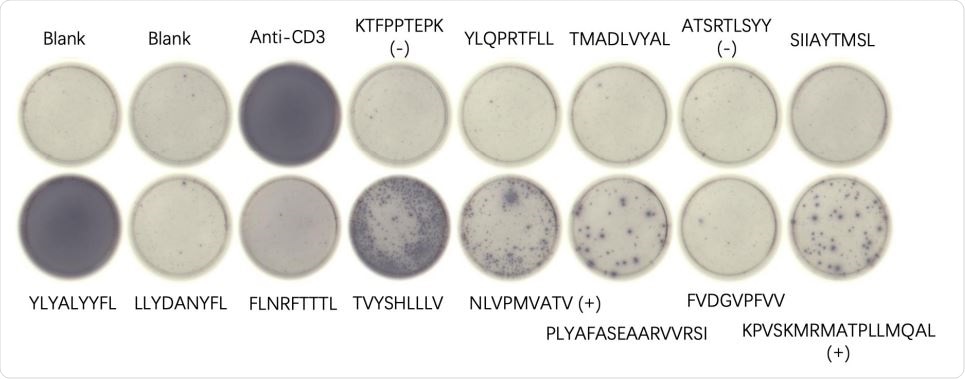
Mouse 3-2 ELISpot results for indicated peptides resulting from an mRNA-LNP vaccine using the ALC-0315 formulation. Blanks contained no added peptides, and positive controls (+) and negative controls (-) are indicated. Anti-CD3 antibody was added as a positive control to the indicated well.
Assembling the vaccine construct
The team used a combined MIRA (Mimicking Intelligent Read Assembly) and machine learning model of peptide-HLA (human leukocyte antigen) immunogenicity to select eight peptides from the SARS-CoV-2 proteome that are predicted to be displayed by the major histocompatibility complex (MHC) class I antigen HLA-A*02:01 that presents antigenic peptides to CD8+ T cells.
Two additional peptides that were not predicted to be immunogenic in HLA-A*02:01 transgenic mice were selected as negative controls.
Three peptides from the SARS-CoV-2 proteome were also optimized for predicted binding to the histocompatibility 2, class II antigen (H-2-IAb) that is involved in the activation of CD4+ T cells.
Using UniProt to assess the peptides’ suitability for protection against variants, the team found that none of the SARS-CoV-2 peptides selected were found to be mutated in the B.1.1.7, B.1.351, or P.1 variants that emerged in the UK, South Africa, and Brazil, respectively.
Next, the vaccine peptides were joined together to form a single polypeptide construct for mRNA--lipid nanoparticle (LNP) delivery. The assembled vaccine construct also included positive control peptides previously shown to be immunogenic in HLA-A*02:01 and H-2-IAb mouse models.
What did they find?
The team found that three of the eight vaccine peptides and the two control peptides were immunogenic in mice transgenic for the human HLA-A*02:01 gene.
The observed immunogenicity of two MHC class I peptides (YLYALVYFL and TVYSHLLLV) and one MHC class II peptide (PLYAFASEAARVVRSI) indicated that the vaccine might provide protection against high titers of SARS-CoV-2.
The researchers say they now plan to test the efficacy of this pan-COVID-19 T cell vaccine in HLA-A*02:01 mice challenged with the South African (B.1.351) variant of SARS-CoV-2.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Gifford D, et al. Preliminary Immunogenicity of a Pan-COVID-19 T Cell Vaccine in HLA-A*02:01 Mice.bioRxiv, 2021. doi: https://doi.org/10.1101/2021.05.02.442052, https://www.biorxiv.org/content/10.1101/2021.05.02.442052v1
- Peer reviewed and published scientific report.
Carter, B., Huang, P., Liu, G., Liang, Y., Lin, P. J. C., Peng, B.-H., McKay, L. G. A., Dimitrakakis, A., Hsu, J., Tat, V., Saenkham-Huntsinger, P., Chen, J., Kaseke, C., Gaiha, G. D., Xu, Q., Griffiths, A., Tam, Y. K., Tseng, C.-T. K., & Gifford, D. K. (2023). A pan-variant mRNA-LNP T cell vaccine protects HLA transgenic mice from mortality after infection with SARS-CoV-2 Beta. Frontiers in Immunology, 14. https://doi.org/10.3389/fimmu.2023.1135815. https://www.frontiersin.org/articles/10.3389/fimmu.2023.1135815/full.