The ongoing coronavirus disease 2019 (COVID-19) pandemic is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a highly contagious RNA virus.
Scientists across the world have been working at record speed to develop vaccines such as Pfizer’s BNT162b2 vaccine and Moderna’s mRNA-1273 vaccine. Various regulatory bodies, including the U.S. Food and Drug Administration (FDA), have granted emergency authorization to these vaccines, which can trigger an effective immune response against SARS-CoV-2.
Research has demonstrated that serum antibody production and neutralizing activity among COVID-19 vaccine recipients differ significantly. Also, the antibodies elicited by some vaccines may not be effective against the newly emerged SARS-CoV-2 variants. Therefore, the effectiveness of these vaccines should be assessed to understand the correlation between the levels of vaccine-induced antibodies and their neutralizing activity against SARS-CoV-2 and the variants.
A new study has been published on the bioRxiv* preprint server which focuses on the correlation between the vaccine-triggered Immunoglobulin G (IgG) levels and neutralization titers against SARS-CoV-2 variants. Further, neutralization activities against the variants (B.1.1.7, B.1.525, and B.1.351), using Pfizer or Moderna vaccine, sera were assessed.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Current Study
The current study was approved by Temple University IRB (IRB #28021), and the researchers have received consent forms from the candidates involved in this study.
Sera samples were collected from thirty individuals who were vaccinated using the Pfizer vaccine, and the samples were obtained between twenty-two to sixty-eight days after the second dose.
Additionally, sera samples from nineteen individuals were obtained, who received the Moderna vaccine, after twenty-four to forty-nine days following the second dose of the vaccine.
The researchers also collected peripheral blood samples three weeks to two months after the second dose of the vaccine. Enzyme-linked immunosorbent assay (ELISA) was used to assess the serum titers of specific IgG antibodies to SARS-CoV-2 spike S1.
The researchers also conducted neutralization assays using recombinant vesicular stomatitis virus (rVSV)–based pseudoviruses that contain S protein of SARS-CoV-2.
The S protein was obtained from the SARS-CoV-2 isolated in Wuhan (reference isolate-Wild Type, WT) and the variants D614G, UK-B.1.1.7, UK-B.1.525, and SA-B.1.351. The team used a four-parameter logistic curve to determine serum neutralizing titers, at 50% inhibitory dilution, ID50, for all the candidates included in this research. For two-group analysis, the Wilcoxon matched-pairs signed-rank test was performed. Geometric mean titers (GMTs) with 95% CI and Pearson’s correlation coefficients were calculated. P ≤ 0.05 was considered statistically significant.
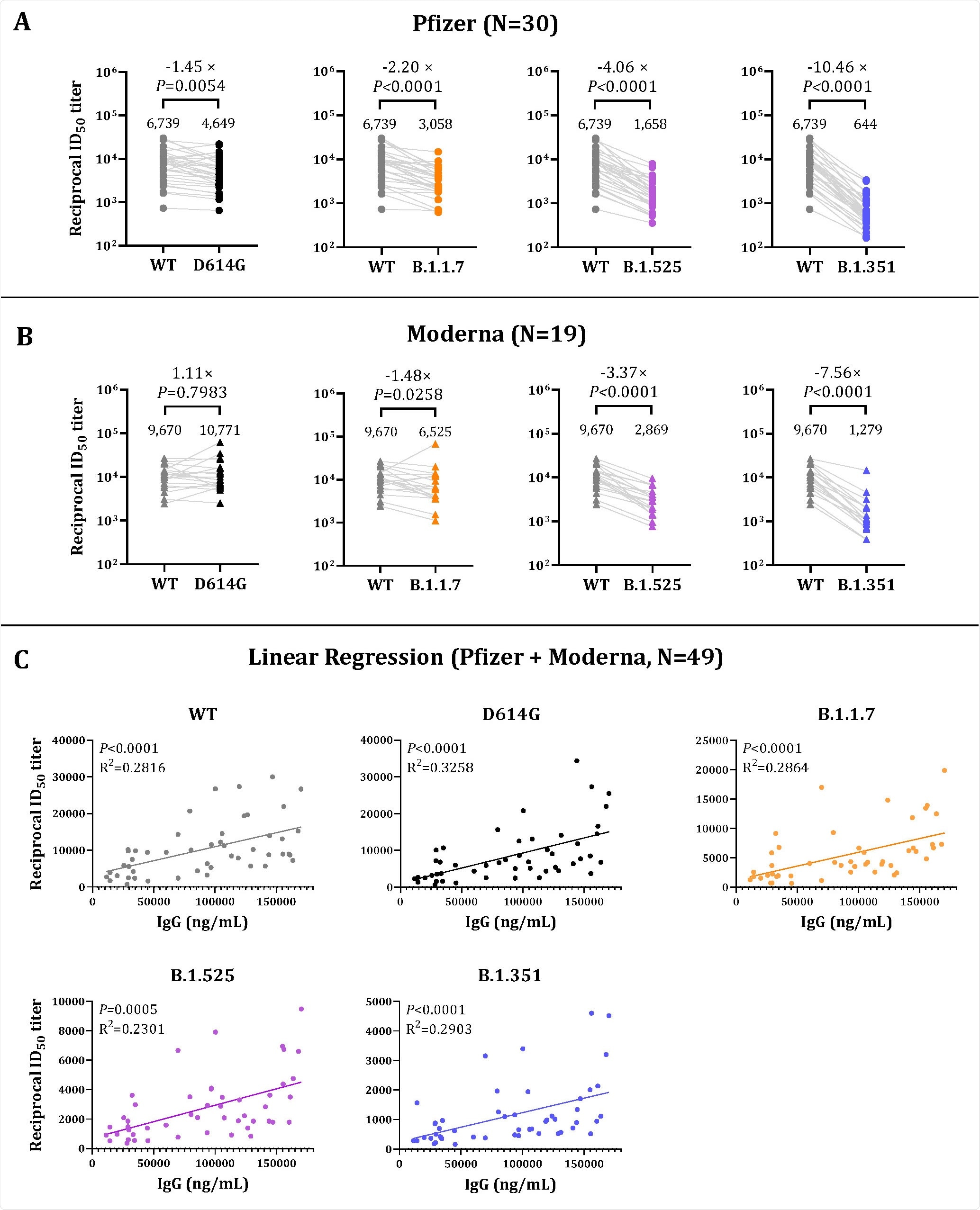
Neutralization of SARS-CoV-2 Pseudoviruses in Sera and its Correlation with Vaccine-elicited IgG Levels. Sera obtained from either Pfzier or Moderna vaccinated subjects were collected three weeks to two months after the second dose vaccine. Neutralizing activity was measured in an assay with recombinant vesicular stomatitis virus (rVSV)–based pseudovirus bearing spike proteins of SARS-CoV-2 WT or the full-set variants. Panels A and B show the reciprocal neutralizing titers at a 50% inhibitory dilution (ID50). The lines connect the WT and variant neutralizing titers in matched samples. Fold changes in the reciprocal serum ID50 in vaccinated sera against the D614G, B.1.1.7, B.1.525, and B.1.351 variants, as compared with WT, are shown above the P value. The dots in Panel A indicate the sera ID50 titers of Pfizer vaccinated subjects; the triangles in Panel B indicate the sera ID50 titers of Moderna vaccinated subjects. The grey, black, orange, purple and blue symbols represent the ID50 titer of the WT, D614G, B.1.1.7, B.1.525, and B.1.351 variant, respectively. The numbers over the dot of each group are the geometric mean titers (GMTs). Panel C shows correlation of the neutralizing titers ID50 (abscissa) and anti-SARS-CoV-2 spike S1 IgG levels (ordinate) of sera from vaccinated subjects. (Pfizer, N=30; Moderna, N=19). In Panel A and Panel B, Wilcoxon matched-pairs signed rank test was used for two-group analysis. In Panel C, linear regression analysis was performed using GraphPad Prism 9.1.1. software. Pearson’s correlation coefficients were calculated. Simple linear regression (solid line) is shown. R2 = goodness of fit. P values less than 0.05 are statistically significant.
A wide-ranging distribution of IgG levels among the vaccinated candidates was observed, which ranged between 11,455 ng/ml (lowest level) and 167,989 ng/ml (highest level). The geometric mean of both the groups, i.e., Pfizer group and Moderna group, were determined. Researchers have calculated the neutralizing infectivity of the pseudoviruses in sera at dilutions ranging from 1:50 to 1:36 to be 450.
The study revealed neutralizing activities in all vaccinated candidates with no statistical difference in serum between the two groups. However, ID50 varied significantly within each group.
Among all the studied variants, B.1.351 showed maximum resistance to the neutralization by sera from both Pfizer (decrease of 10.46-fold) and Moderna (decrease of 7.56- fold) groups. This result is in line with previous studies. Even though there is a decline in the neutralizing titers (GMTs) against all variants, researchers found that sera at ID50 effectively neutralize 99% of both SARS-CoV-2 WT and the variants (D614G, B.1.1.7, B.1.525, and B.1.351).
Additionally, linear regression analysis revealed a strong positive correlation between serum IgG levels and neutralizing activities (ID50) against SARS-CoV-2 WT and SARS-CoV-2 variants.
Some of the limitations of the present study include small sample size and lack of appropriate representativeness. Additionally, the current research lacked live SARS-CoV-2 neutralization assays.
Conclusions
The study revealed that all the participants who were vaccinated using the Moderna or Pfizer vaccine showed effective antibodies against spike proteins of both SARS-CoV-2 WT and the SARS-CoV-2 variants. However, the levels of IgG that are specific against SARS-CoV-2 spike protein S1 and the neutralizing titers (ID50) varied significantly.
A decrease in many folds in GMTs of ID50 was found against SARS-CoV-2 WT. Researchers have reported that sera at a low dilution can effectively neutralize both SARS-CoV-2 and the variants.
Additionally, a positive correlation between serum IgG levels and ID50 titers was observed in both SARS-CoV-2 WT and its variants.
The study authors conclude that a high level of anti-spike IgG provides better protection against SARS-CoV-2 and its variants. Hence, longitudinal monitoring of specific serum IgG levels is necessary to determine the efficacy of vaccines against SARS-CoV-2 and its variants.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Wen-Zhe Ho. et al. (2021). Correlation of vaccine-elicited antibody levels and neutralizing activities against SARS-CoV-2 and its variants, bioRxiv 2021.05.31.445871; doi: https://doi.org/10.1101/2021.05.31.445871, https://www.biorxiv.org/content/10.1101/2021.05.31.445871v1
- Peer reviewed and published scientific report.
Liu, Jinbiao, Brittany H Bodnar, Nigam H Padhiar, Adil I Khan, Fengzhen Meng, Sami Saribas, Peng Wang, et al. 2021. “Correlation of Vaccine‐Elicited Antibody Levels and Neutralizing Activities against SARS‐CoV‐2 and Its Variants.” Clinical and Translational Medicine 11 (12). https://doi.org/10.1002/ctm2.644. https://onlinelibrary.wiley.com/doi/10.1002/ctm2.644.