Researchers in France have suggested that repeated vaccination against severe acute respiratory coronavirus 2 (SARS-CoV-2) may enable previously uninfected individuals to cope with viral variants as efficiently as vaccinees who have recovered from the previous infection.
The SARS-CoV-2 virus is the agent responsible for the COVID-19 pandemic that continues to pose a threat to global public health and has now claimed the lives of more than 3.8 million globally.
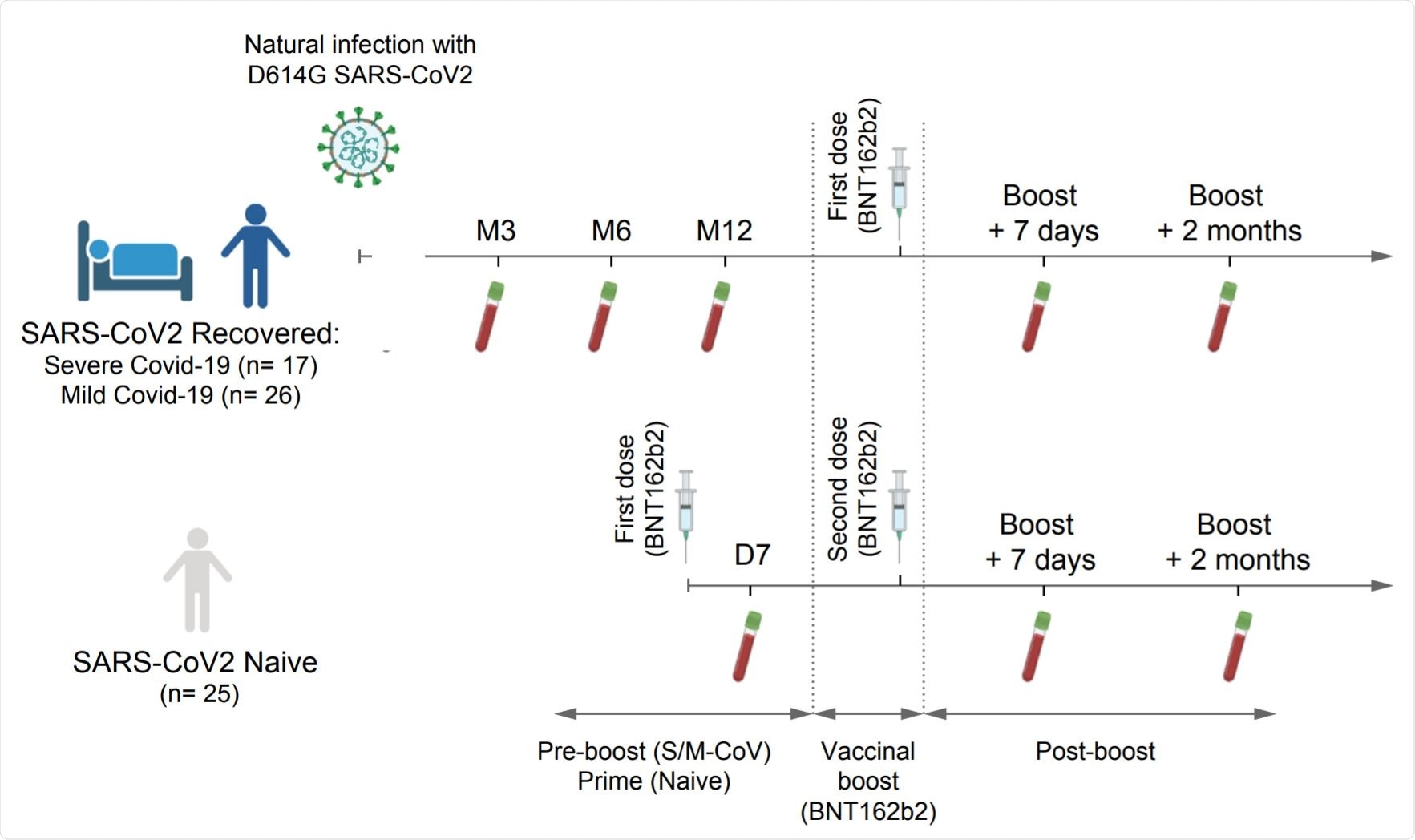
To investigate how previous SARS-CoV-2 infection might shape the memory B cell (MBC) response elicited by vaccination, the team compared previously infected individuals with infection-naïve individuals following immunization with the Pfizer-BioNTech BNT162b2 vaccine.
Matthieu Mahévas from the University of Paris and colleagues found that following vaccination, the MBC pool among recovered patients further matured and harbored potent neutralization capability against variants of concern.
By contrast, maturation of the MBC response following vaccination was much less pronounced among infection-naive individuals. However, half of MBCs directed at the receptor-binding domain (RBD) of the viral spike protein exhibited high affinity for several variants of concern, and one-third retained neutralizing potency against B.1.351 lineage that emerged in South Africa.
The spike RBD mediates the initial stage of the infection process by binding to the human host cell receptor angiotensin-converting enzyme 2 (ACE2).
The team says repeated vaccine challenges could reduce these differences between previously infected and naïve individuals through the recall of affinity-matured MBCs and allow naive vaccinees to cope efficiently with variants of concern.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.
Study. Image Credit: https://www.biorxiv.org/content/10.1101/2021.06.17.448459v1.full.pdf

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
More about the concerns regarding viral variants
Vaccination represents the most promising approach to controlling the COVID-19 pandemic. The Pfizer-BioNTech and Moderna messenger MRNA (mRNA) vaccines that encode the spike protein of the original SARS-CoV-2 strain identified in Wuhan, China are now being deployed on a mass scale worldwide.
Data from both clinical trials and studies conducted in real-world settings have shown the vaccines to be highly effective at both preventing infection and reducing disease severity.
However, the emergence of SARS-CoV-2 variants harboring mutations in key B cell epitopes has raised concerns that the virus will evolve to escape infection- or vaccine-induced immunity.
Several variants of concern contain mutations and deletions that are mainly located in the RBD and the N-terminal domain of the viral spike protein.
The RBD mutations are of particular importance, since a large fraction of the neutralizing antibodies that are elicited following infection or vaccination target this domain.
Although the B.1.1.7 variant that was first identified in the UK exhibits increased transmissibility compared with the wild-type virus, it does not show resistance to neutralizing antibody responses.
By contrast, the B.1351 and P.1 lineages that first emerged in South Africa and Brazil, respectively, have both been shown to reduce neutralization potency among COVID-19 recovered individuals and infection-naive vaccinees.
MBCs provide another layer of protection
Aside from the serum antibody response to SARS-CoV-2, the MBCs generated against the virus provide another layer of immune protection.
MBCs not only persist after infection but continuously evolve and mature by the progressive acquisition of somatic mutations in their variable region genes to improve affinity through an ongoing germinal center response, potentially driven by antigenic persistence,”
Mahévas
These MBCs further drive the recall response following antigenic rechallenge by differentiating into new antibody-secreting cells (ASCs) that display the diverse range of high-affinity-antibodies contained in the MBC repertoire.
However, repeated antigenic stimulation through either vaccination or viral challenge may be deleterious and reduce the diversity of the overall response and the targeting of drifted epitopes, say the researchers.
Understanding how vaccination impacts the memory B cell pool shaped by the previous infection and determining its capacity to neutralize variants is therefore critical.
“More generally, deciphering how MBCs from naive vaccinees differ and evolve in comparison with SARS-CoV-2 recovered patients is also of major importance,” adds the team.
What did the researchers do?
The researchers longitudinally characterized the dynamics, clonal evolution, affinity, and neutralization capacity of anti-SARS-CoV-2 MBC responses following immunization with the Pfizer-BioNTech vaccine among infection-naive individuals and recovered individuals who had been infected six months to one year previously.
What did they find?
The team found that the overall RBD-specific MBC population was remarkably stable for up to 12 months following infection with SARS-CoV-2.
Following vaccination among the recovered patients, the MBC pool selectively expanded, further matured, and harbored potent neutralizing capacity against variants of concern.
The MBC pool contained highly mutated, affinity-matured clones that settled, expanded, and persisted for up to two months after the vaccination boost.
Despite this, vaccination had a limited impact on the diversity of the previously matured repertoire.
“We demonstrate that mRNA vaccination selects high-affinity neutralizing clones, without compromising the overall MBC pool,” writes Mahévas and colleagues.
The MBC pool also progressively matured in the infection-naive vaccinees. However, the maturation and amplification were less pronounced, resulting in fewer RBD-specific MBCs, compared with recovered patients.
The majority of sera from recovered patients efficiently neutralized the B1.351 variant following vaccination, while the infection-naïve vaccines exhibited a significantly weaker neutralizing response.
However, among the naïve vaccinees, a large proportion of B cell clones retained a high affinity for and neutralizing potency against variants of concern, with one-third retaining neutralizing potency against B.1.351.
What did the authors conclude?
“Despite the fact that the amplitude and quality of the MBC response after mRNA vaccine appears to be lower in naive than in previously infected individuals, high-affinity clones with neutralizing potency against VOC settle in their repertoire, suggesting that their MBC pool could compensate for the time-dependent decay of the initial antibody response,” say the researchers.
Our observations suggest that repeated challenges, even against the original spike protein, will compensate for these differences by recall of affinity-matured memory B cells and allow [naïve] vaccinated people to cope efficiently with most variants actually described,”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Mahévas M, et al. Memory B cells control SARS-CoV-2 variants upon mRNA vaccination of naive and COVID-19 recovered individuals. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.06.17.448459
- Peer reviewed and published scientific report.
Sokal, Aurélien, Giovanna Barba-Spaeth, Ignacio Fernández, Matteo Broketa, Imane Azzaoui, Andréa de La Selle, Alexis Vandenberghe, et al. 2021. “MRNA Vaccination of Naive and COVID-19-Recovered Individuals Elicits Potent Memory B Cells That Recognize SARS-CoV-2 Variants.” Immunity 54 (12): 2893-2907.e5. https://doi.org/10.1016/j.immuni.2021.09.011. https://www.sciencedirect.com/science/article/pii/S1074761321003964.