Evaluating an individual’s immunity to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for coronavirus disease 2019 (COVID-19), is required because vaccine responses and/or protection from a previous infection can subside over time. One traditional method that has been used to quantify SARS-CoV-2 neutralizing antibodies levels in plaque reduction neutralization test (PRNT). Despite the utility f this technique, it is laborious, requires biosafety level 3 (BSL3) working conditions due to the use of a live virus, and typically does not provide final results for several days.
 Study: Development and utilization of a surrogate SARS-CoV-2 viral neutralization assay to assess mRNA vaccine responses. Image Credit: 18percengrey / Shutterstock.com
Study: Development and utilization of a surrogate SARS-CoV-2 viral neutralization assay to assess mRNA vaccine responses. Image Credit: 18percengrey / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The neutralizing antibodies that develop during infection with SARS-CoV-2 target the receptor-binding domain (RBD) of the viral spike (S) protein. The RBD plays a role in the entry of SARS-CoV-2 into the host by binding to the angiotensin-converting enzyme 2 (ACE2) receptor that is present on the epithelial cells of the airway. Many monoclonal human antibodies are capable of binding to the spike protein RBD but are not capable of inhibiting infection in vitro.
“Two RBD-specific human monoclonal antibodies have been developed for passive immunization therapy and shown positive therapeutic effects when administered early in the course of COVID-19l.”
In the current study, which was originally published on the preprint server medRxiv*, the authors evaluate several different competitive enzyme-linked immunosorbent assays (ELISAs) that could be used to measure serum antibodies that are capable of blocking RBD-ACE2r binding and can therefore be correlated to live virus neutralization.
About the study
A total of 32 COVID-19 patients were included in the current study, none of whom were hospitalized or experienced severe symptoms of COVID-19. In fact, some of the participants were asymptomatic while infected with SARS-CoV-2.
The surrogate neutralizing antibodies for these patients were already quantified with the help of PRNT. The study also involved 41 individuals post-vaccination and 14 individuals who had recovered from COVID-19 and did not undergo vaccination.
Competitive ELISA was performed using serum samples of individuals who have recovered from COVID-19 as well as in vaccinated individuals. Competitive ELISA of patients with COVID-19 was also carried out and the value was compared with PRNT.
Neutralizing activity was also measured in individuals receiving two different vaccines; namely, the Pfizer-BioNTech and Moderna messenger ribonucleic acid (mRNA) vaccines. Changes in surrogate neutralizing activity were monitored for up to 6 months post-vaccination in several participants.
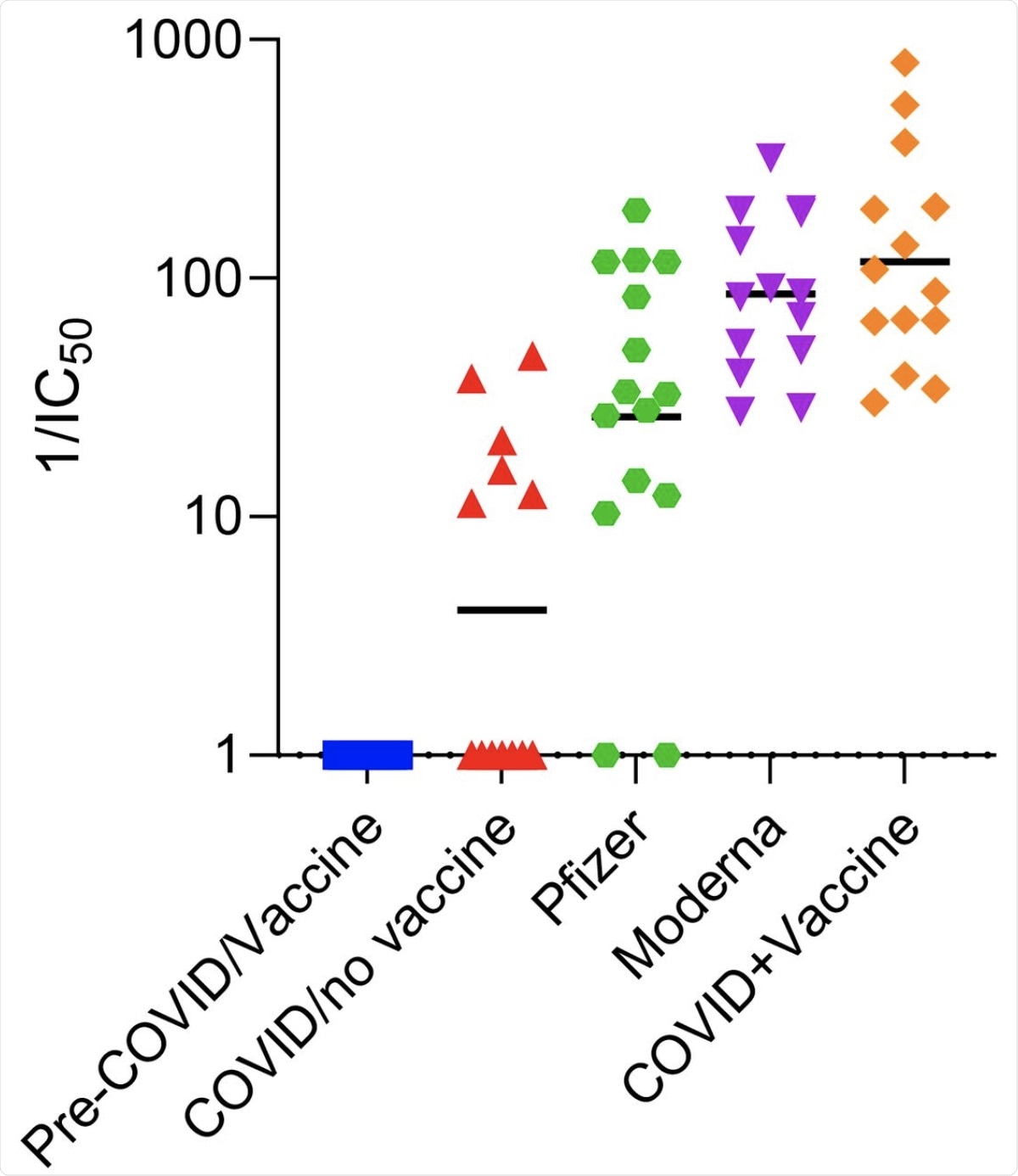 Inhibition of ACE2r binding to RBD by serum from different groups of individuals. The ability of different groups of individuals serum (described in Table 1) to inhibit ACE2r binding to RBD was measured by competitive ELISA and calculated reciprocal IC50 values are shown Y-axis. Data are from subject groups labeled in Table 1 as labeled. Each symbol represents a different person. Mean values are highlighted by bar in graph above and shown in Table 1, along with 95% CI and p values for groups with significant differences.
Inhibition of ACE2r binding to RBD by serum from different groups of individuals. The ability of different groups of individuals serum (described in Table 1) to inhibit ACE2r binding to RBD was measured by competitive ELISA and calculated reciprocal IC50 values are shown Y-axis. Data are from subject groups labeled in Table 1 as labeled. Each symbol represents a different person. Mean values are highlighted by bar in graph above and shown in Table 1, along with 95% CI and p values for groups with significant differences.
Study findings
The study initially faced several technical issues that were eventually overcome by the use of reverse formatted competitive ELISA for the measurement of serum antibody levels.
Taken together, significant differences were observed in the surrogate neutralizing activity of individuals vaccinated after having had COVID-19 as compared to both unvaccinated subjects with a history of COVID-19 and who received different vaccines.
The data also showed a peak that was followed by a slow decline of surrogate neutralizing antibodies from day 35 to day 200 post the first dose of the vaccine. It was also observed that vaccinated individuals with a history of COVID-19 had higher levels of surrogate neutralizing antibodies as compared to those without prior infection. Thus, it can be implied that booster shots or a third exposure can provide additional protection.
This study also demonstrated a decline in surrogate neutralizing antibodies 6 months post-vaccination. This observation is particularly concerning as it demonstrates that the protection conferred by current mRNA vaccines against SARS-CoV-2 infection is waning.
Conclusion
“Use of the present assay may be a feasible approach to assess SARS-CoV-2 protection of larger populations and identifying risk factors for suboptimal response to vaccine or waning responses over time.”
There are certain weaknesses associated with the current study, which include a limited number of subjects per clinical group, a lack of a correlation between providing protection and neutralizing activity, and the lack of participants under the age of 18, whose immune system may be different from the adults.
“Further studies are urgently needed to better understand the heterogeneity and duration of COVID-19 vaccine-induced serum neutralizing responses in larger populations and their relationship to clinically relevant outcomes of SARS-CoV-2 exposure and infection.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Wisnewski, A. V., Liu, J., Lucas, C., et al. (2021). Development and utilization of a surrogate SARS-CoV-2 viral neutralization assay to assess mRNA vaccine responses. medRxiv. doi:10.1101/2021.08.05.21261616 . https://www.medrxiv.org/content/10.1101/2021.08.05.21261616v1 .
- Peer reviewed and published scientific report.
Wisnewski, Adam V., Jian Liu, Carolina Lucas, Jon Klein, Akiko Iwasaki, Linda Cantley, Louis Fazen, Julian Campillo Luna, Martin Slade, and Carrie A. Redlich. 2022. “Development and Utilization of a Surrogate SARS-CoV-2 Viral Neutralization Assay to Assess MRNA Vaccine Responses.” Edited by Paulo Lee Ho. PLOS ONE 17 (1): e0262657. https://doi.org/10.1371/journal.pone.0262657. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0262657.