The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has currently infected over 205 million individuals, with the current number of total deaths from coronavirus disease 19 (COVID-19) standing at over 4.33 million. SARS-CoV-2 drugs are urgently needed for the sake of saving lives and reviving economies around the world. The effectiveness of drugs currently under development, however, is limited by several factors, including poor specificity. While therapeutic antibodies have been identified as having higher specificity against SARS-CoV-2, they are challenging to mass-produce due to high production costs and low yields.
In a research paper recently published in the journal eLife, researchers from several US institutions have developed a nanobody-based candidate drug that effectively prevents and suppresses severe SARS-CoV-2 infection in animals.
If validated in human trials, the drug could present itself as a more suitable candidate for COVID-19 treatment than other therapeutic antibodies due to its thermostability and ability to be produced easily. In addition, using the nanobody drug, infection might be prevented and treated in the short-term.
The Nanosota-1 drug
Globally, scientists are studying the suitability of nanobodies as a candidate for the treatment of COVID-19. Nanobodies are single-domain antibodies derived from heavy-chain-only antibodies found in members of the Camelidae family, such as alpacas and llamas. Although smaller than conventional antibodies, they bind with high affinity and specificity.
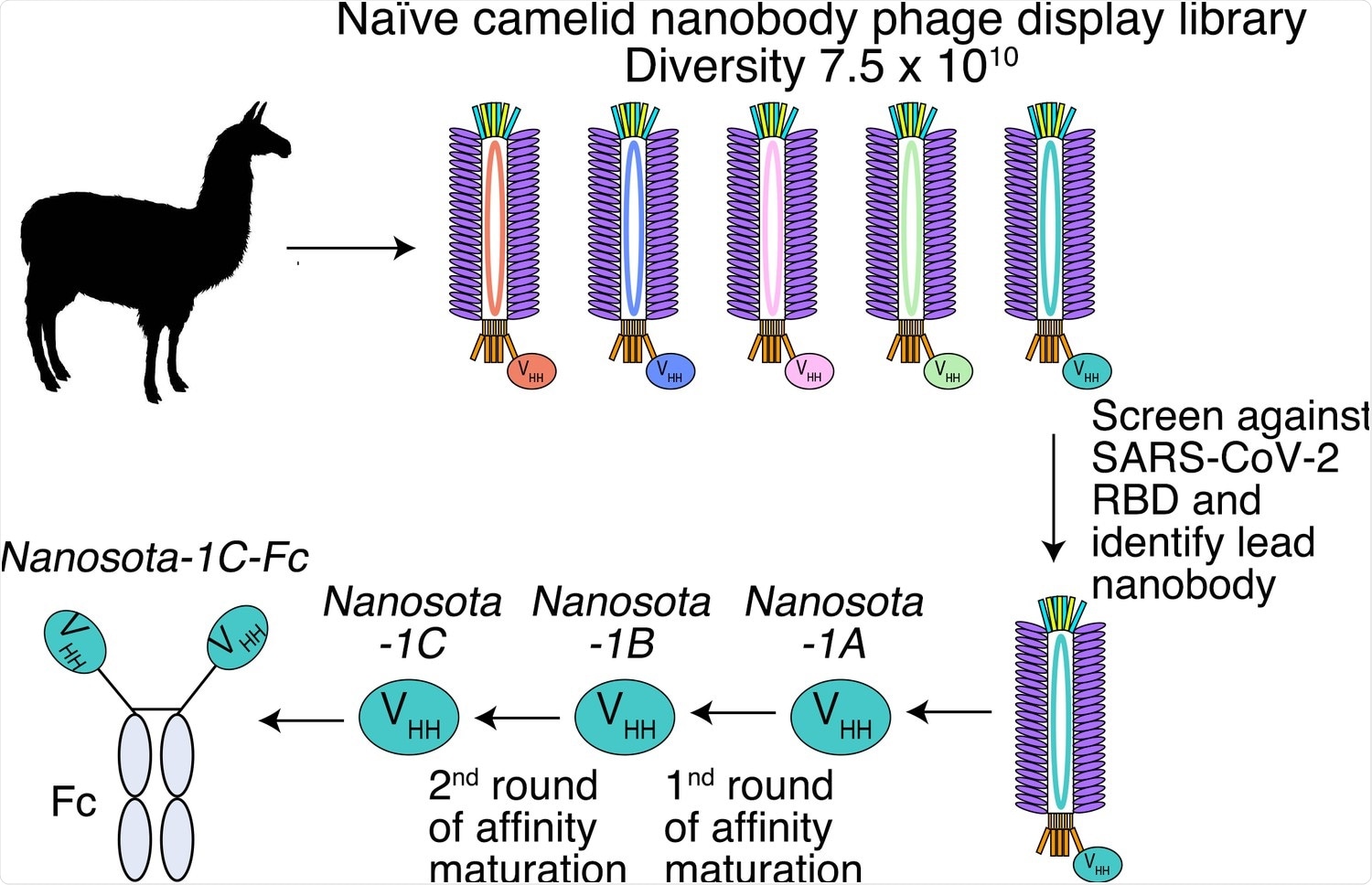
Construction of a camelid nanobody phage display library and use of this library for screening of anti-SARS-CoV-2 nanobodies.
The Nanosota antibodies have been experimentally shown to bind to the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein with high affinity, blocking the binding of the protein to the human angiotensin-converting enzyme 2 (ACE2) and prevent entry into the cells. This principle underpins how the drug Nanosota-1 works, as the drug contains nanobodies that bind to SARS-CoV-2 spike protein to block infection
The study
In the current study, the researchers developed a series of anti-SARS-CoV-2 nanobody drug candidates by screening a nanobody phage display library from camelid species against the viral receptor-binding domain (RBD).
One such candidate was the nanosota-1 series of antibodies, which were found to competitively bind to the SARS-CoV-2 RBD with ACE2 and bind more strongly. Thus, inhibiting SARS-CoV-2 infection by preventing cell entry.
Notably, the best performing and lead drug candidate, Nanosota-1C-Fc, showed preventive and therapeutic effectiveness against SARS-CoV-2 infection in hamster and mice models. Further, the lead candidate showed excellent in vitro stability across temperatures and very good bioavailability and biodistribution compared to other series members. Thus, the Nanosota-1 series are ideal RBD-targeting drug candidates since they suppress and inhibit SARS-CoV-2 viral particles.
“Importantly, we showed that the effectiveness of Nanosota-1C-Fc was not limited to in vitro experiments, but translated directly to in vivo experiments by demonstrating efficacy in animal models,” the researchers explained in the study.
Conclusion
The characteristics of the drug, including excellent in vitro thermostability, high tissue bioavailability, and good in vivo stability, are crucial for implementing the Nanosota-1C-Fc as a potential treatment for COVID-19. Setting it apart from traditional antibody drugs in development, Nanosota-1 can be produced at high yields without compromising its stability across wide temperature ranges. In addition, the nanobody candidate drug can be produced cost-effectively, remaining stable during manufacturing, transport, and storage. Hence, it could help compensate for the shortage of current monoclonal antibody therapies and ultimately aid in treating COVID-19.
Source:
Journal reference: